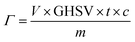Purification of yellow phosphorus tail gas for the removal of PH3 on the spot with flower-shaped CuO/AC†
Zhandong Ren*a,
Shanshan Quana,
Yuchan Zhua,
Liang Chenb,
Wenxing Denga and
Biao Zhanga
aSchool of Chemistry and Environmental Engineering, Wuhan Polytechnic University, Wuhan 430023, P. R. China. E-mail: renzhandong@163.com
bEnvironmental Engineering & Science, Kunming University of Science and Technology, Kunming 650224, P. R. China
First published on 16th March 2015
Abstract
The process of PH3 adsorption removal for purifying yellow phosphorus tail gas on the spot with flower-shaped CuO was investigated in this study. Flower-shaped and irregular-shaped CuO/AC adsorbents were prepared by hydrothermal and impregnation methods, respectively. They can effectively remove PH3 less than 1 mg m−3 and the purification efficiency is nearly 100% without fluctuation. However, the morphology of CuO on the adsorbent surface plays an important role in phosphine adsorption. The breakthrough adsorption capacity of the flower-shaped CuO adsorbent was 96.08 mg(PH3)/g(adsorbent), which was 2.23 times that for irregular-shaped CuO. The purification efficiency of the flower-shaped CuO was also influenced by temperature, oxygen volume fraction and space velocity. At a temperature of 100 °C and an oxygen volume fraction of 1.6%, the adsorption capacity is the best. The adsorbent can be renewed and the regenerated catalyst can also efficiently remove PH3 with a purification efficiency of nearly 100%. In the process of catalytic oxidation, according to XPS, we can conclude that CuO plays a very important role in phosphine adsorption and that oxygen is able to accelerate the oxidation of PH3 and oxidize Cu to regenerate the active species in the process of purification.
1. Introduction
Yellow phosphorus is an important chemical product. There is about 2500–3000 Nm3 of byproduct, tail gas, when producing 1 t of yellow phosphorus.1 The concentration of CO is above 85% (vol ratio) and the caloric value is about 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 kJ m−3 in tail gas.2 At present, most yellow phosphorus manufacturers just use tail gas as a fuel or directly discharge it through torch burning. The burned gases, including greenhouse gas CO2 (as well as phosphate, sulfide, fluoride etc.), are released into the atmosphere and cause significant environmental pollution. At the same time, it is also a great waste of CO gas.
700 kJ m−3 in tail gas.2 At present, most yellow phosphorus manufacturers just use tail gas as a fuel or directly discharge it through torch burning. The burned gases, including greenhouse gas CO2 (as well as phosphate, sulfide, fluoride etc.), are released into the atmosphere and cause significant environmental pollution. At the same time, it is also a great waste of CO gas.
The contents of the different components in the tail gas varied with different processing techniques used in phosphor producing, and the typical content of yellow phosphorus tail gas is as follows:3–5 It mainly consists of CO (85–90 vol%) and other components, such as CO2 (2–4 vol%), N2 (3–5 vol%), H2 (3–5 vol%), CH4 (0.4 vol%), O2 (0.2 vol%), H2S (600–3000 mg m−3), COS (20–2000 mg m−3), CS2 (1–50 mg m−3), P4 and PH3 (500–1000 mg m−3), HF and SiF4 (400–500 mg m−3), and AsH3 (1–8 mg m−3). PH3 is a potent catalyst poison in CO synthesizing chemistry, even at a low concentration.6,7 Moreover, considering its high toxicity and carbon monoxide (CO) resources waste, removal from yellow phosphorus tail gas has become a compelling issue. After completely removing PH3 (less than 5 mg m−3),6,7 yellow phosphorus tail gas can be used as a chemical resource to produce chemical products, such as methanol, methyl formate, dimethyl oxalate, dimethyl carbonate and dimethyl ether.8–12
During the past few decades, a lot of research has focused on removing PH3 by absorption method. Activated carbon can absorb PH3,2,13 but the maximum adsorption capacity was only 12 mg-PH3/g-activated carbon (AC) and cannot reduce the PH3 content to less than 5 mg m−3. In the literature,14–21 a catalytic oxidation method with metal oxide catalysts was adopted to remove COS, CS2 and PH3. The method is simple and offers convenient operation, economical investment and highly efficient removal of phosphors. Copper oxide is commonly used as the active species. Li22 investigated Cu/ZSM-5(Y) zeolite adsorbents and the maximum adsorption capacity was 31 mg-PH3/g-adsorbent. Chang23 prepared a novel sol–gel-derived Cu/TiO2 adsorbent, which exhibited exceptional capacities of 40.62, 49.52, and 108.48 mg-PH3/g-Cu/TiO2 for the oxidative capture of phosphine (PH3) in N2, air, and humidified air, respectively. Yu1 studied Cu/AC absorbents prepared by various copper precursors at different impregnation solution concentrations and calcination temperatures. The biggest PH3 breakthrough adsorbed amount was 112.38 mg-PH3/g-adsorbent. Yang,24 Ning25 and Yi20 prepared a series of Cu/AC with Zn, Ce, Fe, La to further improve the efficiency of PH3 adsorption removal.
However, the effect of the morphology of the absorbent on PH3 adsorption performance has never been studied. In the gas desulfurization, some researchers26 have investigated the importance of morphology. Jung27 found that surface area of the absorbent has a significant effect on the desulfurization performance, and they suggested that surface area contributes to the capacity of the sorbent. Therefore, we think that investigation of the influence of the morphology of absorbents on dephosphorization is quite important and very necessary. In addition, in many studies, PH3 was removed from mixed simulation gas (PH3 + N2), not the actual yellow phosphorus tail gas (PH3 + CO) on the spot. There is a vast difference between the two in the carrier gas, one of which is an inert atmosphere; the other is a reducing atmosphere. In the process of purification, phosphine adsorbed on the activated carbon would be oxidized to form P2O3 or P2O5 by oxygen and these oxidization products could be adsorbed onto activated carbon more easily than PH3. However, the oxygen can also react with CO to form CO2, thus affecting the phosphine oxide adsorption. So, the objective of this research was to study the morphology of CuO/AC absorbents and the purification process parameters in situ for PH3 removal in yellow phosphorus tail gas. This is beneficial for industrial applications.
2. Materials and methods
2.1 Preparation and regeneration of the adsorbent
Active carbon (AC) carrier prepared from a commercial carbon (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) was used as the adsorbent support in this study. First, the AC support was crushed and sieved to 1–2 mm, followed by washing with distilled water, filtration, and drying at 120 °C for 3 h. The irregular-shaped CuO/AC adsorbents were prepared by the impregnation of 2 g of pretreated AC with an aqueous solution of 0.2 mol L−1 Cu(NO3)2 (30 ml). The impregnation was then carried out under stirring for 24 h. These wet samples were dried in a drying cabinet at 120 °C for 3 h followed by calcining in a furnace at a calcination temperature of 400 °C for 2 h (heating rate 5 °C min−1). The flower-shaped CuO/AC adsorbents were prepared by hydrothermal method. Sodium citrate (2 g) and active carbon (2 g) were added to 0.2 mol L−1 CuCl2·2H2O (30 ml) under vigorous stirring. Precipitation was done by dropwise addition of 7.5 mol L−1 sodium hydroxide (2 ml). The obtained suspension was autoclaved at 180 °C for 6 h. The precipitate was then filtered, thoroughly washed with water, and oven dried overnight at 120 °C. The absorbent regeneration experiments were carried out on a catalyst that had failed after purifying tail gas for 250 h. The deactivated CuO/AC catalyst was regenerated in place in the adsorption tower with air oxidation, water vapor washing, alkaline washing, water washing and drying.2.2 Material characterization
X-ray diffraction (XRD) was used to analyze the structures of the flower-shaped and irregular-shaped CuO. The inspection was carried out at room temperature on a D/Max-IIIA diffractometer (Rigaku, Japan), using Cu Kα radiation operating at 40 kV and 30 mA. The surface morphology was characterized by scanning electron microscopy (SEM: S-3000N, Hitachi Co., Japan). Analysis of the composition was carried out using X-ray fluorescence (XRF: EDX-720, Shimadzu, Japan). A Micromeritics ASAP2020 surface area analyzer was used to measure N2 adsorption isotherms at −196 °C. The BET surface area was calculated from the isotherms using the Brunauer–Emmett–Teller (BET) equation.2.3 Adsorption purification process
The tail gas was from the yellow phosphorus production workshop of Yunnan Jianglin Group Phosphide-product Company and the gas composition was close to the typical contents of yellow phosphorus tail gas, as mentioned above. Before coming into the gas adsorption tower, the majority of the hydrogen sulfide in the yellow phosphorus tail gas was removed through alkaline washing. Tail gas with a PH3 content of 300–1000 mg m−3 was sent into the gas adsorption tower. The adsorption tower has an inner diameter of 5 cm and a height of 50 cm (Fig. 1). Evaluation of the adsorbents for dephosphorization was conducted in an upflow fixed-bed with 25.5 cm bed height at atmospheric pressure, 40–110 °C, and a space velocity of 750–1300 h−1, using a tail gas (>90% CO and 0.5–2.0% O2). The tail gas is analyzed from the sampling port at the top of the tower. In this experiment, the contents of PH3 in both the raw material gas and the purification gas are analyzed by a PH3 content detecting-tube (made by Beijing Labor and Protection Institute Technological Developing Company).2.4 Purification efficiency and adsorption capacity
The tail gas purification efficiency is defined as the efficiency with which the harmful impurities are removed from the tail gas. It can be expressed by the following formula:f: purification efficiency, c0: PH3 content in yellow phosphorus tail gas, mg m−3, cf: PH3 content after purification, mg m−3.
Dephosphorization of the absorbent was continued until the breakthrough point. The breakthrough point was defined as the PH3 content of the outlet gas exceeding 5 mg m−3. The adsorption capacity can be expressed by the following formula:
3. Results and discussions
3.1 Crystal structure and morphology
The morphologies of the CuO prepared with the hydrothermal and impregnation methods were observed with FESEM. In Fig. 2, the black areas with big particles in the background are the active carbon carriers and the bright areas are CuO particles. A clear view of the flower-shaped structure obtained using the hydrothermal method can be seen in Fig. 2(a). The leafy nanosheets reveal that the flowers consist of many tiny petals (Fig. 2(b)). The typical length of one petal is about 300–500 nm, while the diameter is in the range of 200–300 nm. The full array of one flower-shaped structure with circumferential symmetry is in the range of 800–1000 nm. The CuO particles prepared by simple impregnation and calcination method were irregular-shaped (Fig. 2(c)). From comparison of Fig. 2(a) and (c), we can conclude that the flower-shaped structure would provide more surfaces and active sites for the reaction. It can also be proved from the BET characterization that the specific surface areas of the flower-shaped and irregular-shaped CuO were 38.15 m2 g−1 and 25.03 m2 g−1, respectively. | ||
| Fig. 2 SEM images of CuO nanostructures with flower-shaped (a and b), irregular-shaped (c) and regenerated flower-shaped CuO (d). | ||
The crystallinity and crystal phases of the flower-shaped and irregular-shaped CuO were examined by X-ray diffraction, as shown in Fig. 3. All the reflections on the XRD pattern could be indexed to the monoclinic CuO phase with lattice constants comparable to the reported data (JCPDS 05-0661). Moreover, the major peaks located at 2θ values of 35.6° and 38.8°, indexed as the (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 11) and (111) planes, respectively, are characteristic of CuO crystallites. In Fig. 3, there are two extra diffraction peaks located at 2θ values of 36.4° and 42.2°, indexed as the (111) and (200) planes of the Cu2O crystal in the irregular-shaped CuO sample, which is due to a small amount of copper that was not completely oxidized in the thermal oxidation preparation process. The Cu2O has poor adsorption of PH3,20,23 so it would reduce its adsorption performance. No other peaks related to other phases and impurities were found in the XRD pattern of the flower-shaped CuO, which were attributed to the hydrothermal preparation process. In addition, the diffraction peak intensity of the irregular-shaped CuO is stronger than that of the flower-shaped CuO, which may be due to the high sintering temperature in the impregnation preparation process. The nominal CuO loading is 19.35 wt% and the actual content loading, which was measured by XRF, is 16.10 wt% for the flower-shaped CuO and 17.42 wt% for the irregular-shaped CuO.
11) and (111) planes, respectively, are characteristic of CuO crystallites. In Fig. 3, there are two extra diffraction peaks located at 2θ values of 36.4° and 42.2°, indexed as the (111) and (200) planes of the Cu2O crystal in the irregular-shaped CuO sample, which is due to a small amount of copper that was not completely oxidized in the thermal oxidation preparation process. The Cu2O has poor adsorption of PH3,20,23 so it would reduce its adsorption performance. No other peaks related to other phases and impurities were found in the XRD pattern of the flower-shaped CuO, which were attributed to the hydrothermal preparation process. In addition, the diffraction peak intensity of the irregular-shaped CuO is stronger than that of the flower-shaped CuO, which may be due to the high sintering temperature in the impregnation preparation process. The nominal CuO loading is 19.35 wt% and the actual content loading, which was measured by XRF, is 16.10 wt% for the flower-shaped CuO and 17.42 wt% for the irregular-shaped CuO.
3.2 Effect of the morphology of CuO on PH3 adsorption removal
As shown in Fig. 4(a), the AC adsorbent without CuO loading showed very poor PH3 adsorption capacity. According to the phosphine removal efficiency curve, the purification efficiency was below 50% after the initial 10 h and the PH3 adsorption capacity was only 1.50 mg(PH3)/g(adsorbent). However, the PH3 removal efficiency was significantly enhanced through CuO loading, particularly with the flower-shaped CuO. At the early stage, the adsorbents were gradually activated and the PH3 purification efficiency gradually increased to 100% after 15 h. At the stable stage, the purification efficiency was almost fixed at 100% without fluctuation, which shows that CuO/AC catalyst has good ability to remove PH3. The adsorption capacity of the flower-shaped CuO adsorbent is 96.08 mg(PH3)/g(adsorbent), which is 2.23 times that of the irregular-shaped CuO. This is partly because the BET specific surface area of the flower-shaped CuO is larger than the irregular-shaped CuO. On the other hand, the morphology of the absorbent would have a great effect on its performance. The comparative calculation of adsorption capacity in relation to overall BET would clarify the differences between both catalysts. In Fig. 4(b), in the unit surface area, the adsorption capacity of the flower-shaped and the irregular-shaped CuO adsorbent is 2.52 and 1.72 mg(PH3)/m2(adsorbent), respectively. Therefore, the morphology of CuO on the adsorbent surface also plays an important role in phosphine adsorption. In addition, from the X-ray diffraction pattern, there is a small amount of Cu2O crystal in the irregular-shaped CuO adsorbent, which is also the reason for the poor adsorption performance.3.3 Effect of oxygen content on PH3 adsorption removal
Fig. 5 illustrates the effect of the oxygen concentration on the PH3 purification performance. Oxygen content is one of the most important factors that influences the PH3 adsorption capacity. Direct oxidation of PH3 with gaseous O2 is inefficient at room temperature. However, adsorbed O2 molecules are able to accelerate the PH3 oxidation and also oxidize the reduced Cu0 metals and Cu+ ions to regenerate the active species for further PH3 capture. As observed in Fig. 5(a), in the absence of O2, the breakthrough of PH3 occurs within approximately 36 h and the adsorption capacity is only 18.70 mg(PH3)/g(adsorbent). Once 0.5 vol% oxygen was introduced into the tail gas, the breakthrough time was increased to 144 h and the adsorption capacity was improved significantly to 74.79 mg(PH3)/g(adsorbent). When the volume fraction of oxygen increased to 1.2% and 1.6%, the adsorption capacities were further expanded to 87.25 and 99.72 mg(PH3)/g(adsorbent), respectively.In Fig. 5(b), the influence of the oxygen concentration is further examined in detail. When the oxygen volume fraction was 0.5 vol% and the purification time was 192 h, the purification efficiency was 94.29% and the mass fraction of PH3 was 20 mg m−3. With the gradual increase of the volume fraction of oxygen, the purifying efficiency improved significantly. When the oxygen contents were 0.9%, 1.2%, and 1.6%, the mass fraction of PH3 and purification efficiency were 16 mg m−3, 6 mg m−3, and 1 mg m−3 and 95.43%, 98.29%, and 99.71%, respectively. If the volume fraction of oxygen increased further, the purification efficiency was stable at nearly 100%. However, too much oxygen would make CO oxidize into CO2 and the excessive residual oxygen would be present in the exhaust gas. It would make the follow-up processing difficult to deal with and bring some security problems, so the optimum oxygen volume fraction is 1.6%. In addition, when the oxygen volume fraction was 1.6%, the purification efficiency was still nearly 100% with the increase of PH3 content from 350 mg m−3 to 900 mg m−3 in the exhaust gas (see Table S1†).
3.4 Effect of adsorption temperature on PH3 adsorption removal
In the adsorption process, the reaction temperature has a great influence on the PH3 purification efficiency of yellow phosphorous tail gas (Fig. 6). When the reaction temperature was 40 °C, the purification efficiency was 98.33% and the content of PH3 in the purified gas was 15 mg m−3. It cannot be used as a raw material gas to produce C1 chemical products, such as ethanol, methyl formate, dimethyl oxalate, dimethyl carbonate and dimethyl ether8–12 because trace levels of phosphine (more than 5 mg m−3) could cause carbonylation catalysts poisoning and expiration.6,7 With increasing adsorption temperature, the content of PH3 decreased and the purification efficiency enhanced. When the adsorption temperature was 100 °C, the purification efficiency was 100% and the content of PH3 in the purified gas was reduced to 0 mg m−3. When the temperature was increased to 120 °C and 140 °C, the PH3 content of the purified tail gas was still less than 1 mg m−3.3.5 Effect of space velocity on adsorption capacity of PH3
The PH3 purification efficiency curves with different amounts of tail gas to be treated are presented in Fig. 7. When the GHSV was increased from 750 h−1 to 1100 h−1 and 1300 h−1 the PH3 purification efficiency still remained at 100%, but the available purification time had reduced to 123 h and 108 h, respectively, from 186 h for 750 h−1. This may be explained by considering that as the GHSV increases, the amount of processing is also raised in unit time, resulting in the reduction of the available tail gas purification time. However, their adsorption capacities were nearly the same, at 96.08, 93.28 and 94.50 mg(PH3)/g(adsorbent), respectively.3.6 Catalyst regeneration
Catalyst regeneration is very important in industrial production. Cyclic use of a catalyst can reduce the production cost and increase its utilization value. Therefore, regeneration experiments were carried out on a catalyst that had failed after 250 h of purification. From the purification curve after regeneration (Fig. 8), it can be seen that the regenerated catalyst can remove PH3 efficiently. The purification efficiency reached 100%, which is the same as the fresh catalyst. The regeneration result indicates that the majority of phosphorus on the CuO adsorbent has been successfully removed through air oxidation, water vapor washing, alkaline washing, water washing and drying. However, the regenerated catalyst's stability was not good enough, especially after 100 h of purification; trace amounts of PH3 (less than 5 mg m−3) could sometimes be detected in the purified gas. In order to determine the reason for the decreased activity, SEM characterization of regenerated CuO was carried out. As shown in Fig. 2(a) and (d), the amount of crystal particles of the regenerated CuO was less than the fresh CuO, perhaps due to the loss of copper in the process of regeneration and the uneven dispersion of CuO particles on the activated carbon. However, the regenerated CuO still retains the flower-structure morphology, which was prepared by hydrothermal method under high temperature and high pressure. Unfortunately, it was observed that some crystal particles had covered on the surface of CuO, which are marked with a red box in the SEM image. This is probably the phosphorus oxide generated in the process of purification that was not removed completely during regeneration. It would reduce the specific surface area, thus decreasing the adsorption efficiency and adsorption capacity. In addition, in the regeneration process, Cu0 and Cu+ were perhaps not fully oxidized into Cu2+, so the capacity is primarily limited by the incompletely oxidized intermediates.3.7 Oxidation and adsorption process
During the adsorption process, direct oxidation of PH3 with gaseous O2 is inefficient. However, a catalytic oxidation reaction can take place in the presence of CuO. PH3 can be oxidized into P2O3 and P2O5 and the adsorptive capacity for P2O3 and P2O5 is far greater than that for PH3. The mechanisms associated with the oxidation process are summarized as follows:Moreover, PH3 is chemisorbed on one of the embedded CuO moieties through P–Cu chelating and can be oxidized into H3PO4, which can be easily absorbed on the active carbon. The process is as follows:
Chang23 put forward the catalytic mechanism.
PH3 → PH2 → H2P–OH → HP(OH)2 → P(OH)3 → HO–P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → H3PO4 O → H3PO4 |
The H+ ion is dissociated from the PH3 to protonate the O2− ion. The chemisorbed PH2 then donates 2 electrons to reduce the bonded Cu2+ into Cu0 while oxidizing itself via hydroxylation to form a P(OH)H2 species. The P(OH)H2 intermediate follows a similar dissociative chemisorption to react with the next CuO until the PH3 becomes P(OH)3 and H3PO4.
To clarify the reaction between the CuO and the PH3, we analyzed the chemical states and chemical environments of the Cu and P species using XPS. Fig. 9(a) shows the Cu 2p XP spectra of the CuO both before and after the capture of PH3. Before adsorption, the Cu 2p3/2 peak centered at 933.42 eV and 935.56 eV can be assigned to CuO and Cu(OH)2. The as-prepared CuO sample contained two satellite peaks at the binding energy (BE) of 944.4 eV and 963.6 eV, clearly indicating Cu 2p ions. After the capture of PH3, the Cu 2p3/2 peak shifted to 932.66 eV. This indicates that the Cu exists in a chemical state other than the CuO form, which is Cu2O or Cu. The change in the peak intensity of the CuO adsorbents before and after adsorption is due to the phosphorus deposition that results from the adsorption and subsequent oxidation of phosphine on the CuO adsorbent. In addition, the satellite peaks disappearing also indicate that the Cu species was reduced. Fig. 9(b) shows the P 2p XP spectra of the CuO sample after the capture of PH3. According to the literature,23 the peaks at 130.3, 132.8, and 133.7 eV can be ascribed to P, P3+(P2O3), and P5+(P2O5 and H3PO4), respectively, because they stayed either in a stable closed shell or in a half-filled configuration. The fresh adsorbent has no phosphorus species, so the P2O3, P2O5 or H3PO4 species appearing in the exhausted sample were generated by an oxidation process. According to the above catalytic mechanism, we can conclude that CuO plays a very important role in phosphine adsorption and oxygen is able to accelerate the PH3 oxidation and oxidize Cu to regenerate the active species in the process of purification.
4. Conclusions
The process of PH3 adsorption removal for purifying yellow phosphorus tail gas on the spot was investigated in this study. The morphology of CuO on the adsorbent surface plays an important role in phosphine adsorption. The flower-shaped CuO/AC adsorbent can effectively remove PH3 to leave less than 1 mg m−3 without fluctuation and the adsorption capacity is 96.08 mg(PH3)/g(adsorbent), which is 2.23 times that of the irregular-shaped CuO. The purification efficiency was also influenced by oxygen volume fraction. Oxygen is able to accelerate the PH3 oxidation and oxidize Cu to regenerate the active species in the process of purification. When the adsorption temperature was above 100 °C, the purification efficiency was 100% and the content of PH3 in the purified gas was reduced to 0 mg m−3. The adsorbent can be renewed and the regenerated catalyst can also efficiently remove PH3 with a purification efficiency of nearly 100%. During the catalytic oxidation adsorption process, PH3 can be oxidized into P2O3 or P2O5, and CuO plays a very important role in phosphine adsorption and oxidation.Acknowledgements
This work was supported by the Natural Science Foundation of Hubei Province (2012FFB04803) and the National Natural Science Foundation of China (31101370).References
- Q. F. Yu, P. Ning, H. H. Yi, X. L. Tang, M. Li and L. P. Yang, Sep. Sci. Technol., 2012, 47, 527–533 CrossRef CAS.
- L. P. Ma, P. Ning, Y. Y. Zhang and X. Q. Wang, Chem. Eng. J., 2008, 137, 471–479 CrossRef CAS PubMed.
- P. Ning and B. N. Ren, Yunnan Environ. Sci., 2003, 22, 149–151 Search PubMed.
- H. P. Gao, P. Ning, C. F. Wu and M. X. Ma, J. Wuhan Univ. Technol., Mater. Sci. Ed., 2010, 2, 53–57 CrossRef PubMed.
- Z. H. Wang, M. Jiang, P. Ning and G. Xie, Ind. Eng. Chem. Res., 2011, 50, 12194–12202 CrossRef CAS.
- X. Q. Wang, P. Ning, Y. Shi and M. Jiang, J. Hazard. Mater., 2009, 171, 588–593 CrossRef CAS PubMed.
- R. Quinn, T. A. Dahl, B. W. Diamond and B. A. Toseland, Ind. Eng. Chem. Res., 2006, 45, 6272–6278 CrossRef CAS.
- C. J. Yoo, D. W. Lee, M. S. Kim, D. J. Moon and K. Y. Lee, J. Mol. Catal. A: Chem., 2013, 378, 255–262 CrossRef CAS PubMed.
- S. Goodarznia and K. J. Smith, J. Mol. Catal. A: Chem., 2012, 353–354, 58–66 CrossRef CAS PubMed.
- Z. N. Xu, J. Sun, C. S. Lin, X. M. Jiang, Q. S. Chen, S. Y. Peng, M. S. Wang and G. C. Guo, ACS Catal., 2013, 3, 118–122 CrossRef CAS.
- J. Q. Wang, J. Sun, C. Y. Shi, W. G. Cheng, X. P. Zhang and S. J. Zhang, Green Chem., 2011, 13, 3213–3217 RSC.
- K. G. Mbuyi, M. S. Scurrell, D. Hildebrandt and D. Glasser, Top. Catal., 2012, 55, 1261–1268 CrossRef.
- Q. F. Yu, M. Li, P. Ning, H. H. Yi and X. L. Tang, Sep. Sci. Technol., 2014, 49, 2366–2375 CrossRef CAS.
- D. He, H. H. Yi, X. L. Tang, P. Ning, K. Li, H. Y. Wang and S. Z. Zhao, J. Mol. Catal. A: Chem., 2012, 357, 44–49 CrossRef CAS PubMed.
- Y. C. Liu and H. He, J. Phys. Chem. A, 2009, 113, 3387–3394 CrossRef CAS PubMed.
- P. Ning, K. Li, H. H. Yi, X. L. Tang, J. H. Peng, D. He, H. Y. Wang and S. Z. Zhao, J. Phys. Chem. C, 2012, 116, 17055–17062 CAS.
- L. Wang, S. D. Wang and Q. Yuan, Fuel Process. Technol., 2010, 91, 777–782 CrossRef CAS PubMed.
- X. Q. Wang, J. Qiu, P. Ning, X. G. Ren, Z. Y. Li, Z. F. Yin, W. Chen and W. Liu, J. Hazard. Mater., 2012, 229–230, 128–136 CrossRef CAS PubMed.
- H. H. Yi, D. He, X. L. Tang, H. Y. Wang, S. Z. Zhao and K. Li, Fuel, 2012, 97, 337–343 CrossRef CAS PubMed.
- H. H. Yi, Q. F. Yu, X. L. Tang, P. Ning, L. P. Yang, Z. Q. Ye and J. H. Song, Ind. Eng. Chem. Res., 2011, 50, 3960–3965 CrossRef CAS.
- H. H. Yi, S. Z. Zhao, X. L. Tang, P. Ning, H. Y. Wang and D. He, Catal. Commun., 2011, 12, 1492–1495 CrossRef CAS PubMed.
- W. C. Li, H. L. Bai, J. N. Hsu, S. N. Li and C. C. Chen, Ind. Eng. Chem. Res., 2008, 47, 1501–1505 CrossRef CAS.
- S. M. Chang, Y. Y. Hsu and T. S. Chan, J. Phys. Chem. C, 2011, 115, 2005–2013 CAS.
- L. P. Yang, H. H. Yi, X. L. Tang, P. Ning, Q. F. Yu and Z. Q. Ye, J. Rare Earths, 2010, 28, 322–325 CrossRef.
- P. Ning, H. H. Yi, Q. F. Yu, X. L. Tang, L. P. Yang and Z. Q. Ye, J. Rare Earths, 2010, 28, 581–586 CrossRef CAS.
- M. Pineda, J. M. Palacios, F. Tomas, C. Cilleruelo, E. Garcia and J. V. Ibarra, Energy Fuels, 1998, 12, 409–415 CrossRef CAS.
- S. Y. Jung, H. K. Jun, S. J. Lee, T. J. Lee, C. K. Ryu and J. C. Kim, Environ. Sci. Technol., 2005, 39, 9324–9330 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra00578g |
| This journal is © The Royal Society of Chemistry 2015 |