Influence of gas-to-liquid (GTL) fuel in the combined blend of Jatropha biodiesel and diesel: an analysis of engine combustion–performance–emission parameters†
H. Sajjad*,
H. H. Masjuki*,
M. Varman,
M. A. Kalam,
M. I. Arbab,
S. Imtenan and
H. K. Rashedul*
Centre for Energy Sciences, Department of Mechanical Engineering, Faculty of Engineering, University of Malaya, 50603 Kuala Lumpur, Malaysia. E-mail: h.sajjad013@gmail.com; masjuki@um.edu.my; mdrashedhasan82@gmail.com; Fax: +60 3 79675317
First published on 6th March 2015
Abstract
This study reports the production of Jatropha biodiesel (JBD) and a comparative analysis of the fuel properties, engine performance and emission characteristics of blends of JBD (J20) and GTL fuel (G20) with diesel, including a combined blend of JBD, GTL and diesel (DJG20). The ternary blend was selected to combine the promising properties of the two alternative fuels. In this study, a four-cylinder compression ignition engine was used to perform tests at different speeds under constant torque. DJG20 and G20 showed the most promising properties among all tested fuels. In combustion analysis, the peaks of both in-cylinder pressure and heat release rate (HRR) of G20 were lower and occurred at later crank angles compared to those of diesel. The other two blends demonstrated higher peaks of both parameters, while DJG20 showed lower peaks than J20 in both cases. The peak locations of J20 and DJG20 were slightly higher than those of diesel. The engine performance results showed an average increase in brake thermal efficiency (BTE) and lower fuel consumption (BSFC) for G20, whereas the other two blends exhibited decreases in BTE but increases in BSFC compared to diesel. The emission analysis results of all fuel blends showed lower CO, HC and smoke emissions than diesel. For NOx emissions, G20 showed a significantly decreased value, whereas the other two blends showed increased values compared to diesel. Compared to J20, DJG20 showed improvements in all performance–emission parameters.
1 Introduction
Worldwide awareness of the energy crisis related to the dwindling of fossil fuel reserves and the heinous environmental effects associated with these fuels has led to the exploration of the alternative energy carriers. Biodiesel and gas-to-liquid (GTL) fuel can be considered as prospective future transportation fuel. Biodiesel is designated as the mono-alkyl esters of fatty acids, which can be extracted from vegetable oils, animal fats and alcohol. Biodiesel has special features like renewability, biodegradability and non-toxicity. When compared to fossil diesel, biodiesel possesses a higher cetane number (CN) and flash point, has inherent lubricity and demonstrates more diminution in emissions.1,2 Jatropha curcas can be regarded as a potential feedstock for biodiesel production because of its non-edible origin, higher oil yield compared to other non-edible feedstocks and the compliance of the biodiesel yielded from its crude oil with the US ASTM D6751 and European Union EN 14214 biodiesel standards.3,4 GTL fuel can be synthesized in a number of ways such as the methane reforming process, Fischer–Tropsch (FT) synthesis and hydrocracking processes.5,6 The FT synthesis converts a mixture of carbon monoxide and hydrogen into various liquid hydrocarbons by using suitable catalysts.7 GTL fuel possesses higher CN, virtually zero sulfur content and negligible amounts of aromatic compounds;8–10 it also has significantly lower emissions than diesel and biodiesel.11–13 Thus, it can be regarded as a clean alternative fuel that can yield low exhaust emissions without any major engine modifications or significant losses in efficiency.Several studies have been reported in recent years regarding the blends of diesel–biodiesel and diesel–GTL. Most of these studies14–19 showed that biodiesel blends with diesel resulted in decreases in power and CO, HC and smoke emissions, whereas increases in fuel consumption and NOx emissions were observed. Sahoo et al.19 studied the performance and emission characteristics of JBD blends (20%, 50% and 100% by vol.) with diesel in a CI tractor engine. The results showed lower power, HC and smoke opacity but higher CO and NOx emissions of the blends compared to those of diesel. Sahoo and Das20 also studied the combustion characteristics of the same blends of JBD. The results revealed that JBD blends showed higher peak values of in-cylinder pressure, but lower HRR peak values than those of diesel. Mofijur et al.3 tested JBD blends (10% and 20% by vol.) with diesel in a single-cylinder diesel engine. The study showed lower brake power and torque, reduced CO and HC emissions and higher BSFC and NOx for the blends compared to those of reference fuel diesel. Studies on GTL–diesel blends reported that blending GTL fuel with diesel can certainly improve the fuel properties of the blends, which lead to better engine performance and exhaust emissions compared to diesel.8–10 Wu et al.21 investigated GTL–diesel blends in a six-cylinder turbocharged direct-injection diesel engine at different load-speed conditions. The results indicated improvements in BTE and fuel consumption along with simultaneous decreases in CO, HC, smoke and NOx emissions. Ng et al.22 and Schaberg et al.23 used GTL fuel blended with ULSD and EN590 diesel, respectively, in diesel-fueled passenger cars and observed good reductions in exhaust emissions.
Some recent studies have reported blends combining two alternative fuels (GTL fuels or biodiesels) with diesel with the aim of further improving the fuel properties and engine performance results. Sanjid et al.24 studied two combined blends of palm–Jatropha biodiesel with diesel in a multi-cylinder diesel engine at different engine speeds. In engine performance tests, PBJB5 and PBJB10 biodiesels showed higher BSFC values (7.55–9.82%) and marginally lower output power, while emission tests revealed lower CO (about 9.53–20.49%) and HC (about 3.69–7.81%) emissions compared to diesel fuel. Habibullah et al.25 studied combined blends (PB15CB15) of palm–coconut biodiesel with diesel in a single-cylinder diesel engine and compared the performance of that blend with individual blends (30% by vol.) of palm (PB30) and coconut (CB30) biodiesel with diesel. PB15CB15 showed an improvement in brake power along with reductions in BSFC and NOx emissions compared to CB30. When compared to PB30, PB15CB15 showed significant reductions in CO and HC emissions and improved BTE. Although only a few studies on combined blends of two biodiesels with diesel have been reported, to date, no study has been performed using blends combining biodiesel, GTL fuel and diesel.
Regarding the blend ratios of biodiesel with diesel, previous studies16–18,26–29 have indicated that a 15–25% blend of biodiesel with petroleum diesel demonstrates comparatively better engine performance than any other blend ratio, and the EU has aimed to use 20% biodiesel in blends with diesel by 2020.30 All of the previous studies regarding biodiesel–diesel and GTL–diesel blends discussed the test results of the individual blend and compared them with those of reference fuel diesel or within two biodiesels. A comparative analysis among blends of GTL–diesel and biodiesel–diesel has not yet been performed. In addition, combined blends of two alternative fuels with diesel can improve the performance and emission features when compared to their individual blend with diesel. An analysis of the studies concerning combined blends of alternative fuels showed that the effect of the addition of GTL fuel in these combined blends is still uninvestigated. Moreover, in most previous studies, the engine test condition was either variable speed or variable load. Hence, the performance and emission parameters of engine testing using these blends also require further investigation under constant torque conditions.
The objectives of this study are to improve engine performance and emission characteristics by using a combined blend of non-edible biodiesel (JBD), GTL fuel and diesel and comparing it to traditional blends (20% by vol.) of biodiesel–diesel and GTL–diesel. This study of the combined blend will ensure the existing emission benefits of biodiesel along with the improved fuel properties and engine performance–emission parameters of GTL fuel and diesel.
2 Experimental setup and procedures
2.1 Biodiesel production
Jatropha curcas crude oil has an FFA content of 8% (acid value 16 mg KOH per g); thus, it should first undergo acid-catalyzed esterification to reduce the FFA level.31 Subsequently, an alkali-catalyzed transesterification process can be applied. At first, crude Jatropha oil was placed in a favorite jacket reactor with a 1 liter capacity equipped with an IKA Eurostar digital model stirrer and a Wiscircu water bath arrangement. Next, methanol (in a molar ratio of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and H2SO4 (1% v/v) were added. The system was controlled at a temperature of 50–60 °C for 3 h by the circulation of hot water through the jacket while stirring at 1100 rpm. To check the change in FFA level, 5 mL samples were collected at 10 min intervals. This process was continued until the FFA level decreased to 1–2%. After the completion of acid-esterification, the mixture was poured in a separation funnel to isolate the esterified product and catalyst layer. The catalyst layer contained excess H2SO4 and alcohol and was positioned as the upper layer. After isolation, the lower layer was extracted and placed into a rotary evaporator to eliminate excess methanol and water. The yield of this process is approximately 98%.32 After the completion of acid-esterification, the yielded product was subjected to alkali-catalyzed transesterification.
1) and H2SO4 (1% v/v) were added. The system was controlled at a temperature of 50–60 °C for 3 h by the circulation of hot water through the jacket while stirring at 1100 rpm. To check the change in FFA level, 5 mL samples were collected at 10 min intervals. This process was continued until the FFA level decreased to 1–2%. After the completion of acid-esterification, the mixture was poured in a separation funnel to isolate the esterified product and catalyst layer. The catalyst layer contained excess H2SO4 and alcohol and was positioned as the upper layer. After isolation, the lower layer was extracted and placed into a rotary evaporator to eliminate excess methanol and water. The yield of this process is approximately 98%.32 After the completion of acid-esterification, the yielded product was subjected to alkali-catalyzed transesterification.
In the alkali-catalyzed trans-esterification process, methanol (25% v/v oil) and potassium hydroxide 1% (w/w oil) were added with esterified Jatropha oil in a biodiesel reactor. This mixture was stirred at 1000 rpm at a temperature of 60 °C for 3 h. Subsequently, the mixture was placed into a separation funnel, where it separated into two layers: glycerol-impurities (lower layer) and methyl esters (upper layer). Eventually, the lower layer was isolated, and the upper layer was cleansed thoroughly by spraying 50% (v/v) hot (50 °C) distilled water over the methyl esters while shaking gently to ensure proper washing. The cleansing process was applied repeatedly until the methyl esters reached a pH of 7. To discard excess water and methanol, vacuum distillation was applied to the methyl esters using an IKA RV10 rotary evaporator. To remove moisture from the methyl esters, anhydrous sodium sulfate was used. Finally, the methyl esters were filtered with high-quality filter papers.
2.2 GC analysis and fatty acid composition of fuels
Gas chromatography (GC) analysis was performed to investigate the fatty acid composition of the produced biodiesel. The detailed specifications of the instrument and operation conditions are presented in this section. A 1 μL sample of JBD was injected into a Shimadzu GC-2010A series chromatography panel equipped with a flame ionization detector and a BPX70 capillary column (30.0 m × 0.25 μm × 0.32 mm, inner diameter). An initial temperature of 140 °C was set for 2 min and then increased to 200 °C at a rate of 8 °C min−1. The temperatures of the oven, injector and the detector ports were set at 140, 240 and 260 °C, respectively. The carrier gas (helium) was passed through the column at a rate of 1.10 mL min−1 with a split ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Each peak was identified by comparison with an external standard reference mixture of fatty acid methyl esters. The percentage of each methyl ester was calculated based on this value, and the results are presented in section 3.1.
1. Each peak was identified by comparison with an external standard reference mixture of fatty acid methyl esters. The percentage of each methyl ester was calculated based on this value, and the results are presented in section 3.1.
2.3 Fuel property testing and engine test setup
In this study, three blends were prepared as sample fuels. JBD and GTL fuel were mixed with diesel in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. Thus, each sample blend contained 80% diesel and 20% biodiesel or GTL fuel. The blends containing JBD and GTL fuel were designated as J20 and G20, respectively. The third blend (DJG20) contained 50% diesel, 30% JBD and 20% GTL fuel. The kinematic viscosity and density were measured by using an SVM 3000-automatic viscometer, manufactured by Anton Paar, UK. A Pensky-Martens flash point-automatic NPM 440 was used to measure flash point. The calorific value was measured by an automatic C2000 basic calorimeter. An automatic NTE 450 Cloud and Pour point tester was used to measure CP and PP values. The oxidation stability was measured using an 873 Rancimat automatic machine.
4. Thus, each sample blend contained 80% diesel and 20% biodiesel or GTL fuel. The blends containing JBD and GTL fuel were designated as J20 and G20, respectively. The third blend (DJG20) contained 50% diesel, 30% JBD and 20% GTL fuel. The kinematic viscosity and density were measured by using an SVM 3000-automatic viscometer, manufactured by Anton Paar, UK. A Pensky-Martens flash point-automatic NPM 440 was used to measure flash point. The calorific value was measured by an automatic C2000 basic calorimeter. An automatic NTE 450 Cloud and Pour point tester was used to measure CP and PP values. The oxidation stability was measured using an 873 Rancimat automatic machine.
A four-cylinder, four-stroke, water-cooled diesel engine was used for experimental investigation. The compression ratio of the 2477 cm3 diesel engine was 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the bore × stroke was 91.1 × 95 mm with turbo-charged aspiration, and the injection pressure was 157 bar. The rated power was 65 kW at 4200 rpm. The combustion chamber was swirl-type, and a distributor-type injection pump was used in the fuel systems. A radiator-type cooling system and pressure feed lubrication system were used in the test rig. The test engine was directly coupled to a Froude-Hoffman AG250 eddy current dynamometer. A schematic of the test rig is depicted in Fig. 1. The initial engine run was performed with diesel before starting the tests with G20, J20 and DJG20 fuels. Before starting the test with fuel blends, the engine was run for ten minutes to ensure the removal of the residual diesel. After the test of each sample fuel, the fuel line was purged with diesel to remove that sample and ready it for the next sample. This procedure was applied for testing in all test conditions. The operations were performed at the same injection timing for all tested fuels.
1, the bore × stroke was 91.1 × 95 mm with turbo-charged aspiration, and the injection pressure was 157 bar. The rated power was 65 kW at 4200 rpm. The combustion chamber was swirl-type, and a distributor-type injection pump was used in the fuel systems. A radiator-type cooling system and pressure feed lubrication system were used in the test rig. The test engine was directly coupled to a Froude-Hoffman AG250 eddy current dynamometer. A schematic of the test rig is depicted in Fig. 1. The initial engine run was performed with diesel before starting the tests with G20, J20 and DJG20 fuels. Before starting the test with fuel blends, the engine was run for ten minutes to ensure the removal of the residual diesel. After the test of each sample fuel, the fuel line was purged with diesel to remove that sample and ready it for the next sample. This procedure was applied for testing in all test conditions. The operations were performed at the same injection timing for all tested fuels.
In this study, engine tests were performed at constant torque (80 N m) while varying the speed from 1000 rpm to 3000 rpm at intervals of 500 rpm. All of the tests were performed under steady-state conditions with adequately warmed-up exhaust gas and water coolant temperature. To maintain accuracy, each test point was repeated three times, and the mean value was obtained for plotting. In addition, every test data series (i.e., test points with the same fuel type and at various engine speeds) was recorded on the same day to minimize substantial day-to-day variation in the experimental results. To measure the fuel flow rate, a positive-displacement-type flow meter (KOBOLD ZOD) was installed. To record the engine test data, an REO-dCA data acquisition system was incorporated. For exhaust emission analysis, an AVL DICOM 4000 gas analyzer was used to measure the concentrations of CO, HC and NOx. To measure CO and HC, a non-dispersive infrared method was used, whereas NOx was measured electrochemically. For smoke measurement, opacity was measured with an AVL Di-Smoke 4000 using the photodiode detector principle. All emissions were measured during steady state engine operation.
Although the experimental results were not consistently reproducible, from day-to-day, the observed trends were consistent throughout and are in good agreement with data from the same tests performed on different days. This indicates that the effects on combustion characteristics can be reliably analyzed using this test system. To investigate combustion phenomena, the test rig was equipped with adequate sensors. For the measurement of in-cylinder pressure data, a pressure sensor (Kistler 6058A type) was installed in the swirl chamber using a glow plug. To ensure the accuracy of data recording, an amplifier (Kistler 2614B type) that could feed an amplified output of the pressure sensor to a data acquisition system (DAS) was used. A high-precision and robust incremental encoder from Leine & Linde was selected to determine the TDC position and the adjacent signal from the crank angle in each rotation. A DEWE-30-8-CA data acquisition unit was installed for concurrent samplings of the in-cylinder pressure and encoder signals.
2.4 Combustion parameter analysis
To analyze the combustion phenomena of the test fuels, the experimental setup was equipped with the required sensors and a data recorder system, as mentioned in section 2.3. To eliminate variability in cycle-to-cycle data, 100 consecutive combustion cycles of pressure data were recorded, and the average value was then considered in the analysis of the sample fuels in each test. In addition, the Savitzky–Golay33 smoothing-filtering tool was used to reduce the noise effects on the average pressure data. MATLAB® R2009a software was used to calculate the heat release rate (HRR) and start of combustion (SOC).2.5 Accuracies and uncertainty analysis
Uncertainties in any experimental procedure occur depending on the experimental conditions, instrument calibration, observation, data input, test assembly, etc. Therefore, uncertainty analysis is an important technique to validate the accuracy of the experimental results. In this experiment, the percentages of uncertainty in measured quantities such as CO, HC, NOx, and smoke contents were calculated using the percentage uncertainties of the various instruments used. In addition, the relative uncertainty in calculating BSFC was determined using the linearized approximation method of uncertainty.34 The accuracies of the pressure sensor, crank angle encoder, CO, HC, NOx, and smoke opacity were ±10 KPa, ±0.125°, 0.01 vol.%, ±1 ppm vol., ±1 ppm vol. and 0.1%, respectively. The uncertainty values of the referred parameters and BSFC are ±0.5, ±0.03, ±0.01 vol.%, ±1 ppm, ±1 ppm, ±0.5% and ±0.33 g kW−1 h−1, respectively.3 Results and discussion
3.1 Fuel property analysis
Fuel property analysis had been conducted as a part of an effort to predict the qualities of sample fuel blends prior to combustion, performance and emission testing. The fatty acid composition of the produced biodiesel revealed that JBD contains saturated methyl esters (about 24.3%), mono-saturated methyl esters (about 42.6%) and poly-unsaturated methyl esters (about 33.1%). The fuel properties of the produced biodiesel and GTL are listed in Table 1. In this table, saponification number (SN), iodine value (IV) and cetane number (CN) were calculated from the fatty acid composition results and the following empirical eqn (1–3),35 respectively:
 | (1) |
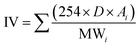 | (2) |
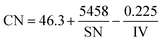 | (3) |
| Properties | ASTM D6751 | Crude Jatropha oil | JBD | GTL |
|---|---|---|---|---|
| Density @ 40 °C (g cm−3) | Not specified | 918.9 | 878.8 | 761.9 |
| Kinematic viscosity @ 40 °C (mm2 s−1) | 1.9–6.0 | 34.072 | 4.2684 | 2.7417 |
| Flash point (°C) | >130 | 210.5 | 176.5 | 103.5 |
| Calorific value (MJ kg−1) | Not specified | 39.420 | 40.899 | 46.785 |
| Cetane number | ≥47 | — | 53.5 | 75 |
| Acid value (mg KOH per g) | <0.50 | 16 | 0.18 | <0.01 |
| Saponification number | Not specified | — | 192.6 | N/A |
| Iodine value | Not specified | — | 93.8 | 1.22 |
| Oxidation stability at 110 °C (h) | >3 | 1.2 | 8.41 | 49.26 |
Miscibility is a significant term to consider when studying binary or ternary fuel blends. A number of previous studies2,36–38 have already ensured that GTL fuel is completely miscible in biodiesel and diesel. Table 2 shows the tested fuel properties of the test fuels. Excessive density of any fuel yields higher viscosity; this has a significant influence on spray atomization efficiency and results in poor combustion along with the formation of engine deposits and higher exhaust emissions.9 Among the test fuels, J20 and DJG20 showed higher density and viscosity compared to diesel, whereas G20 showed lower values of these two parameters. J20 and DJG20 demonstrated respective increases of about 14.4% and 5.86% in kinetic viscosity compared to diesel, whereas the kinetic velocity of G20 was 1.66% lower than that of diesel. DJG20 showed a 2.98% lower value than J20. All of the sample blends fulfill the ASTM D7467 specification. A lower kinematic viscosity of fuel ensures less resistance while it flows through the fuel system and also leads to better fuel atomization.39 Hence, better combustion efficiency was observed for G20 and DJG20 compared to J20, which ultimately resulted in better performance and emission characteristics.
| Properties | Diesel | J20 | G20 | DJG20 |
|---|---|---|---|---|
| Density kg m−3 | 829.6 | 835.1 | 815.8 | 830.1 |
| Kinematic viscosity at 40 °C (mm2 s−1) | 3.07 | 3.35 | 3.03 | 3.25 |
| Calorific value | 44.46 | 43.406 | 45.026 | 43.603 |
| Cetane number | 49 | 58 | 65 | 61 |
| Flash point (°C) | 69.5 | 79.5 | 83.5 | 95.5 |
| CP (°C) | 8 | 8 | 8 | 7 |
| PP (°C) | 7 | 3 | 6 | 6 |
| Oxidation stability at 110 °C (h) | 59.1 | 36.75 | 48.25 | 48.95 |
The flash point maintains an inverse relationship with fuel volatility.40 Higher flash point ensures the safety of fuel for handling and storage, and prevents unexpected ignition during combustion. As JBD primarily consists of methyl palmitate, methyl stearate, methyl oleate and methyl linoleate, it demonstrates a higher flash point than diesel and meets the ASTM D6751 specification of 130 °C. Compared to diesel, G20, J20 and DJG20 showed higher flash points by about 20.1%, 14.4% and 37.41%, respectively. Compared to J20, DJG20 exhibited about a 20.13% increase in flash point. All of these test fuels meet the ASTM D7467 specification for fuel blends. In case of calorific value, J20 and DJG20 exhibited respective calorific values of about 2.37% and 1.93% lower than that of diesel, whereas G20 showed a 1.27% higher calorific value compared to diesel. The higher calorific value of any fuel is desirable because it favors heat release during combustion and improves engine performance.9
CN is a measure of a fuel's auto-ignition quality characteristics. JBD and other blends possess higher CN values than diesel and also meet the ASTM D6751 specification of CN ≥ 47. All of the blends used in this experiment also meet the ASTM D7467 specification. DJG20, J20 and G20 showed respective CN values of approximately 24.49% 14.4% and 32.6% higher compared to diesel. DJG20 showed an approximately 7.14% higher CN than J20.
Due to its saturated fatty acid esters, the oxidation stability of JBD was 8.41 h, meeting the ASTM D6751 as well as the EN 14214 standard specifications. All of the blends showed increased oxidation stability compared to diesel. The oxidation stability values for DJG20, J20 and G20 were 48.95, 36.98 and 48.5 h, respectively, which meet the ASTM D7467 specification.
3.2 Combustion analysis
DJG20 and J20 showed higher peak pressures than diesel fuel, which can be attributed to the combined effects of higher CN, higher BSFC values and the advancement of the start of injection (SOI) timings of the biodiesel.41–43 The higher bulk modulus of biodiesel initiates the advancement of the nozzle opening, resulting in earlier injection compared to diesel.44
In case of G20, the peak in-cylinder pressure was quite lower and occurred at an advanced crank angle compared to those of diesel and the other blends. This can be explained by the higher CN of GTL fuel, which induced a substantially shorter ignition delay, resulting in a decreased premixed combustion zone.21 Thus, a decrease was observed in the peak combustion pressure in the cylinder. It can be deduced that the decreased maximum pressure of G20 can initiate a smooth combustion, which can decrease combustion noise.45
 | (4) |
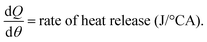
V and 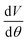 were obtained from the eqn (5) and (6), respectively:
were obtained from the eqn (5) and (6), respectively:
 | (5) |
 | (6) |
Here,  ,
, 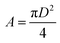 , r = 0.5 × stroke, l = connecting rod length, r = crank radius, D = cylinder bore, and Vc = clearance volume.
, r = 0.5 × stroke, l = connecting rod length, r = crank radius, D = cylinder bore, and Vc = clearance volume.
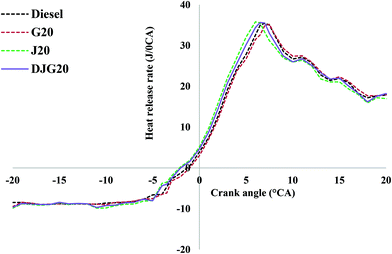
Fig. 3 illustrates the HRR values of diesel and the other test fuels. All sample fuels demonstrated a prompt premixed burning, which led towards the diffusion combustion zone. From the HRR diagrams, it can be observed that the SOC of the biodiesel occurred earlier than that of diesel due to the earlier SOI timing. As the test engine had a pump-line nozzle injection system, fuels with higher densities and higher bulk moduli of compressibility demonstrated advanced SOI times. Thus, the combustion features in this study are explained using SOCs in lieu of ignition delay. The SOC values were obtained from the HRR vs. crank angle diagram. The SOC timings for diesel, J20, G20 and DJG20 are −3.5° ATDC, −4.75° ATDC, −2.75° ATDC and −3.75° ATDC, respectively. The earlier SOC timing of the biodiesel blend also explains the slight increase in peak in-cylinder pressure compared to the other test fuels. The peak values of HRR produced by diesel, J20, G20 and DJG20 were 35.48 J/°CA, 35.82 J/°CA, 35.23 J/°CA and 35.65 J/°CA, respectively, and occurred at 7.25° ATDC, 6.5° ATDC, 7.75° ATDC and 6.75° ATDC, respectively.
The premixed combustion stages of the J20 and DJG20 blends were quite higher and sharper compared to diesel, which also contributed to the higher peaks of in-cylinder pressure with respect to diesel. For G20, the peak value of HRR during premixed combustion was much lower, and the duration of this phase was shorter compared to diesel; however, in the diffusion burning zone, the peak was higher, and the duration was longer compared to diesel. The higher CN of GTL fuel causes a smaller ignition delay, which decreases both the mass of injected fuel and the rate of evaporation of fuel prior to ignition.21,45 As a result, G20 demonstrated a lower burning rate and a smaller amount of energy released during the premixed combustion phase. Since the amount of energy released during the premixed combustion phase was smaller, the diffusion-controlled combustion exhibited more energy.9,10 Due to the lower boiling point of GTL fuel, it promptly vaporizes and mixes with the in-cylinder air, which results in faster diffusion mixing along with a higher rate of diffusion combustion. Thus, a higher peak value of diffusion-burning rate was observed for G20.
3.3 Engine performance test
The improvement in BSFC for the G20 blend can be explained by the combustion phenomena and fuel characteristics. As the fuel was delivered on a fixed volumetric basis, the amount of fuel injected in a single stroke was same for all fuels. Since the G20 contains a higher calorific value, it required a comparatively small quantity of fuel per stroke to produce the same power compared to the diesel and two other blends.49,50 In addition, G20 demonstrated a lower in-cylinder pressure and a lower pressure rise rate, which could assist in compensating for mechanical losses, leading to better combustion.45 The higher BSFC values of the other blends can be ascribed to the volumetric effect of the constant fuel injection rate associated with their higher kinematic viscosity values. Several studies16,51 also confirmed that the fuel consumptions of these blends increase with decreasing calorific value.
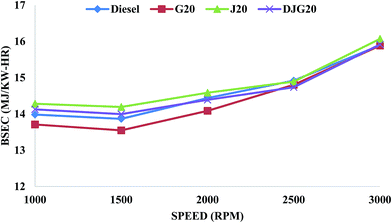
Another significant performance parameter is the brake specific energy consumption (BSEC). Generally, BSEC is introduced to compare the performance of fuels with different calorific values. It can be defined as the product of the BSFC and the calorific value of the fuel. BSEC indicates the amount of energy consumed to produce a unit output power in one hour. Usually, the value of BSEC decreases with increasing energy consumption efficiency. Fig. 5 illustrates the variation in the BSEC values of diesel, G20, J20 and DJG20. J20 and DJG20 blends showed higher BSEC values, while G20 showed a lower value compared to diesel. On average, the BSEC values of J20 and DJG20 were higher by about 1.26% and 0.08%, respectively, compared to that of diesel. In comparison to J20, the average improvement of DJG20 is about 1.18%. For G20, the test results revealed a BSEC value approximately 1.48% lower than that of diesel. The overall improvement in the BSFC and BSEC parameters of DJG20 compared to J20 can be attributed to the presence of GTL fuel, which improved the density and kinematic viscosity of the combined blend.
 | (7) |
Fig. 6 illustrates the variation in the BTE values of diesel, G20, J20 and DJG20. In all test modes, J20 and DJG20 blends showed lower BTE values, while G20 showed higher BTE values compared to diesel. As predicted, all of the tested fuels showed higher BTE under the medium-speed conditions compared to under low-speed operation. Since lower fuel consumption is required to overcome the mechanical losses associated with engine during medium-higher speed operating zone compared to the lower speed zone. At the top dead center, a higher level of spontaneous premixing occurs, which induces a faster rate of combustion.45 At higher speed, all fuels demonstrated lower BTE. This can be attributed to insufficient air, causing incomplete combustion of the fuel.16 On average, J20 and DJG20 showed decreased BTE values by about 1.29% and 0.12%, respectively, when compared to that of diesel. Compared to J20, the BTE of DJG20 showed an increase of about 1.18%. For G20, the test results revealed an approximately 1.61% increase in BTE compared to diesel. This trend is also supported by other studies.21,45 The lower BTE values of the other two blends can be ascribed to the combined effect of their lower calorific values and higher kinetic viscosities. The slight improvement in BTE for DJG20 compared to J20 can be attributed to the presence of GTL fuel in this blend.
Considering efficient energy consumption, the improved BTE of the G20 synthetic fuel blend is a significant advantage. Moreover, its higher brake thermal efficiency is beneficial to the automobile manufacturer as improved BTE widens the range of opportunities to comply with the upcoming strict pollutant regulations and after-treatment system requirements by modifying the injection parameters.
3.4 Exhaust emission test
Fig. 7 illustrates the variation in the CO emission values of diesel, G20, J20 and DJG20. All the blends showed lower CO emissions than diesel, and among the blends, G20 showed the greatest emission reduction compared. On average, DJG20, J20 and G20 showed decreases in CO emissions of approximately 18.96%, 14.76% and 26.13%, respectively, compared to the reference fuel diesel. Compared to J20, DJG20 showed an approximately 4.69% lower CO emission.
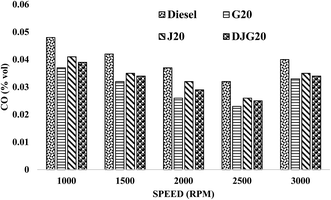 | ||
| Fig. 7 Variation in CO emission for all test fuels within the test speed range under constant torque. | ||
The CO emission reduction of G20 can be explained by the fuel properties and combustion phenomena. G20 exhibited good thermal efficiency (as described in section 3.3.2), which resulted in increased air–fuel ratio. The improved combustion of G20 is attributed to the fuel characteristics such as a higher hydrogen-to-carbon ratio, higher CN and very low aromatic content, which contributed to the reduction in CO. The higher CN of G20 induces the shortening of the ignition delay, which prevents the formation of less over-lean zones. In addition, the lower distillation temperature of GTL fuel induces rapid vaporization, which reduces the probability of flame quenching and thus ensures lower CO emission.21,45 In the case of the other two blends, the lower CO emissions can be explained by the combined effect of the high oxygen content and higher CN.52 Higher CN results in a short ignition delay, producing better combustion. The short ignition delay can also result from the longer chain length of biodiesel, thus improving the combustion process.1 High oxygen content ensures proper in-cylinder temperature, which also facilitates complete combustion.53,54 In the case of DJG20, the combined presence of GTL fuel and JBD resulted in a greater reduction in CO emission compared to diesel and J20.
Fig. 8 illustrates the variation in the HC emission values of diesel, G20, J20 and DJG20. All fuels showed lower HC emission values than diesel. Overall, G20 showed the greatest emission reduction compared to all fuels. On average, the HC emission values of DJG20, J20 and G20 were decreased by approximately 16.28%, 15.16% and 24.48%, respectively, compared to that of the reference fuel diesel. When compared to J20, DJG20 showed an approximately 2.75% lower HC emission.
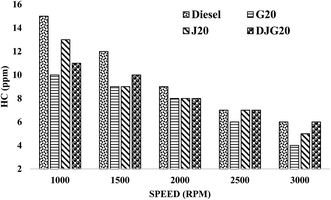 | ||
| Fig. 8 Variation in HC emission for all test fuels within the test speed range under constant torque. | ||
As for CO emission, the reductions in HC emission can be explained by the fuel properties and combustion phenomena of GTL fuel. The higher CN of GTL fuel shortens the ignition delay, which prevents the formation of over-lean regions. The lower distillation temperature of GTL ensures the proper pace of evaporation and mixing with air to constitute a more effective combustible charge, which results in less unburned HC in the exhaust.21,55 For the other two blends, the inherently higher oxygen content of biodiesel induced some advantageous conditions such as post-flame oxidation and higher flame speed during air–fuel interaction, especially in fuel-rich regions. This ensured the proper oxidation of the unburned HC, resulting in the significant reduction in HC emission.43 For DJG20, the combined presence of GTL fuel and JBD yielded a greater reduction in HC emission compared to diesel and J20.
Fig. 9 illustrates the variation in the emission values of diesel, G20, J20 and DJG20. The J20 and DJG20 blends demonstrated higher NOx emissions, whereas G20 showed lower NOx emission values compared to diesel. Among all tested fuels, G20 showed the lowest NOx emission. On average, the NOx emission values of DJG20 and J20 were increased by about 2.63% and 5.43%, respectively, compared to diesel. When compared to J20, DJG20 showed an approximately 2.77% lower NOx emission. For G20, the test results revealed an approximately 7.19% decrease in NOx emission compared to diesel.
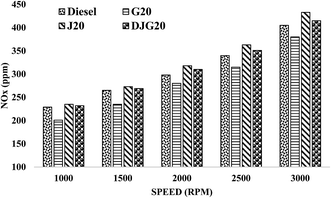 | ||
| Fig. 9 Variation in NOx emission for all test fuels within the test speed range under constant torque. | ||
The decreased NOx emission of G20 can be attributed to the influence of fuel properties on combustion phenomena and exhaust emission. The higher CN of G20 induced a shorter ignition delay followed by a lesser premixed charge, which resulted in a lower combustion temperature and pressure.21,45 These conditions resulted in less thermal NOx formation. The significantly lower aromatic content of GTL fuel also influenced G20, resulting in a lower local adiabatic flame temperature and thus assisting in NOx reduction.49,57 Several studies have revealed that NOx emissions in biodiesel and diesel–biodiesel blends demonstrated increased NOx emissions with increasing unsaturation percentage and decreasing chain length.58,59 For the other two blends, higher NOx emissions were observed in all test modes because of their high oxygen contents and a higher “premixed parts” during combustion, when NOx is primarily formed.60 For DJG20, the presence of GTL fuel in this combined blend resulted in the additional reduction of NOx content in exhaust emission compared to J20.
Fig. 10 illustrates the variation in the smoke emission values of diesel, G20, J20 and DJG20. All of the sample fuels showed lower smoke emission values than diesel. Overall, G20 showed a greater emission reduction compared to J20, DJG20 and diesel. On average, the smoke emission values of DJG20, J20 and G20 were decreased by approximately 8.09%, 4.79% and 9.74%, respectively, compared to that of the reference fuel diesel. Compared to J20, DJG20 showed an approximately 3.49% lower smoke emission.
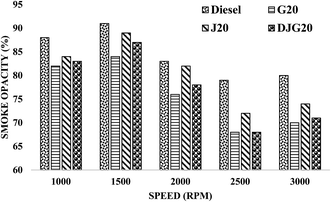 | ||
| Fig. 10 Variation in smoke opacity for all test fuels within the test speed range under constant torque. | ||
This reduction in smoke emission for G20 is in accordance with reports in the literature2,45,62,63 and can be attributed to the combined effect of the absence of aromatics (regarded as soot predecessors), low sulfur content and higher hydrogen-to-carbon ratio of GTL fuel. For the other two blends, the higher oxygen content associated with the lower sulfur content and impurities can explain the decreases in smoke emission.61 For DJG20, the incorporation of GTL fuel and JBD with diesel resulted in an additional reduction in smoke emission compared to diesel and J20.
4 Conclusion
In this study, JBD was produced, and three blends (J20, G20 and DJG20) were used to comparatively investigate the fuel properties, combustion, engine performance and exhaust emissions. The produced JBD showed improved fuel properties compared to the crude oil, and J20 demonstrated further improvements relative to JBD. Regarding density and viscosity, J20 and DJG20 showed higher values than diesel, whereas G20 exhibited lower values. In the case of calorific value, only G20 showed a higher value compared to diesel; J20 and DJG20 had lower values. All of the blends showed higher CN values and flashpoints compared to diesel. Combustion analysis demonstrated that compared to those of diesel, the peak values for both in-cylinder pressure and HRR were higher for J20 and DJG20 but lower for G20. Compared to J20, DJG20 showed lower peak values of these two parameters. The engine performance results demonstrated higher BSFC and BSEC values for J20 and DJG20, whereas G20 showed lower values of these two parameters. In case of BTE, G20 showed higher values compared to diesel, whereas J20 and DJG20 showed lower values. The emission analysis results revealed that all fuel samples showed lower values of CO, HC and smoke compared to diesel. In the case of NOx emission, only G20 showed a reduction compared to diesel, whereas the other blends showed increases. A detailed analysis of the outcome of this study suggests the heightened possibility for the commercial application of J20, G20 and DJG20 blends. These fuel blends may comply with the upcoming strict emission regulations and also contribute to better engine performance features, which are highly valued by automobile manufacturers.Abbreviations
| J20 | 20% JBD + 80% Diesel |
| G20 | 20% GTL + 80% Diesel |
| DJG20 | 50% Diesel + 30% JBD + 20% GTL |
| SN | Saponification number |
| IV | Iodine value |
| PPM | Parts per million |
| RPM | Rotation per minute |
| °CA | Degree crank angle |
| J/°CA | Joules per degree crank angle |
| FT | Fischer–Tropsch |
| SOI | Start of injection |
| SOC | Start of combustion |
| HRR | Heat release rate |
| JBD | Jatropha curcas biodiesel |
| GTL | Gas-to-liquid fuel |
| BTE | Brake thermal efficiency |
| BSFC | Brake-specific fuel consumption |
| BSEC | Brake-specific energy consumption |
| CO | Carbon monoxides |
| HC | Hydrocarbons |
| NOx | Nitrogen oxides |
| CN | Cetane number |
| FFA | Free fatty acid |
| GC | Gas chromatography |
Acknowledgements
The authors would like to acknowledge the University of Malaya for financial support through a research grant (grant number: FP051-2014B), and also a High Impact Research grant titled: “Clean Diesel Technology for Military and Civilian Transport Vehicles” (grant number UM.C/HIR/MOHE/ENG/07).References
- J. Xue, T. E. Grift and A. C. Hansen, Renewable Sustainable Energy Rev., 2011, 15, 1098–1116 CrossRef CAS PubMed.
- M. Lapuerta, O. Armas, J. J. Hernández and A. Tsolakis, Fuel, 2010, 89, 3106–3113 CrossRef CAS PubMed.
- M. Mofijur, H. Masjuki, M. Kalam and A. Atabani, Energy, 2013, 55, 879–887 CrossRef CAS PubMed.
- P. Rao, World Acad. Sci. Eng. Tech., 2011, 75, 855–868 Search PubMed.
- O. P. R. van Vliet, A. P. C. Faaij and W. C. Turkenburg, Energy Convers. Manage., 2009, 50, 855–876 CrossRef CAS PubMed.
- S. S. Gill, A. Tsolakis, K. D. Dearn and J. Rodríguez-Fernández, Prog. Energy Combust. Sci., 2011, 37, 503–523 CrossRef CAS PubMed.
- O. O. James, B. Chowdhury, M. A. Mesubi and S. Maity, RSC Adv., 2012, 2, 7347–7366 RSC.
- A. J. Velaers and S. D. Goede, SAE Int. J. Fuels Lubr., 2012, 5(3), 1174–1186, DOI:10.4271/2012-01-1692.
- H. Sajjad, H. H. Masjuki, M. Varman, M. A. Kalam, M. I. Arbab, S. Imtenan and S. M. A. Rahman, Renewable Sustainable Energy Rev., 2014, 30, 961–986 CrossRef CAS PubMed.
- S. H. Park, D. Lee and C. S. Lee, Proc. Inst. Mech. Eng., Part D, 2014, 228, 85–93 CrossRef CAS PubMed.
- G. Moon, Y. Lee, K. Choi and D. Jeong, Fuel, 2010, 89, 3840–3846 CrossRef CAS PubMed.
- O. Armas, K. Yehliu and A. L. Boehman, Fuel, 2010, 89, 438–456 CrossRef CAS PubMed.
- M. Oguma, S. Goto, K. Oyama, K. Sugiyama and M. Mori, SAE Tech. Pap., 2002 DOI:10.4271/2002-01-1706.
- C. Carraretto, A. Macor, A. Mirandola, A. Stoppato and S. Tonon, Energy, 2004, 29, 2195–2211 CrossRef CAS PubMed.
- H. Aydin and H. Bayindir, Renewable Energy, 2010, 35, 588–592 CrossRef CAS PubMed.
- E. Buyukkaya, Fuel, 2010, 89, 3099–3105 CrossRef CAS PubMed.
- J. Song and C. Zhang, Proc. Inst. Mech. Eng., Part D, 2008, 222, 2487–2496 CrossRef.
- M. Gumus and S. Kasifoglu, Biomass Bioenergy, 2010, 34, 134–139 CrossRef CAS PubMed.
- P. Sahoo, L. Das, M. Babu, P. Arora, V. Singh, N. Kumar and T. Varyani, Fuel, 2009, 88, 1698–1707 CrossRef CAS PubMed.
- P. Sahoo and L. Das, Fuel, 2009, 88, 994–999 CrossRef CAS PubMed.
- T. Wu, Z. Huang, W.-G. Zhang, J.-H. Fang and Q. Yin, Energy Fuels, 2007, 21, 1908–1914 CrossRef.
- H. Ng, R. Carlson and M. Wang, SAE Tech. Pap., 2008, 01–1810 Search PubMed.
- P. Schaberg, J. Botha, M. Schnell, H.-O. Hermann, N. Pelz and R. Maly, SAE Tech. Pap., 2005 DOI:10.4271/2005-01-2187.
- A. Sanjid, H. Masjuki, M. Kalam, S. Rahman, M. Abedin and S. Palash, J. Cleaner Prod., 2014, 65, 295–303 CrossRef CAS PubMed.
- M. Habibullah, H. Masjuki, M. Kalam, I. R. Fattah, A. Ashraful and H. Mobarak, Energy Convers. Manage., 2014, 87, 250–257 CrossRef CAS PubMed.
- M. Canakci, A. N. Ozsezen, E. Arcaklioglu and A. Erdil, Expert Syst. Appl., 2009, 36, 9268–9280 CrossRef PubMed.
- B. S. Chauhan, N. Kumar and H. M. Cho, Energy, 2012, 37, 616–622 CrossRef CAS PubMed.
- S. Jindal, B. Nandwana, N. Rathore and V. Vashistha, Appl. Therm. Eng., 2010, 30, 442–448 CrossRef CAS PubMed.
- S. Godiganur, C. Suryanarayana Murthy and R. P. Reddy, Renewable Energy, 2009, 34, 2172–2177 CrossRef CAS PubMed.
- M. Mofijur, H. Masjuki, M. Kalam, M. Hazrat, A. Liaquat, M. Shahabuddin and M. Varman, Renewable Sustainable Energy Rev., 2012, 16, 5007–5020 CrossRef PubMed.
- M. Y. Koh and T. I. Mohd Ghazi, Renewable Sustainable Energy Rev., 2011, 15, 2240–2251 CrossRef CAS PubMed.
- I. Rizwanul Fattah, H. Masjuki, M. Kalam, M. Wakil, A. Ashraful and S. Shahir, Energy Convers. Manage., 2014, 83, 232–240 CrossRef CAS PubMed.
- A. Savitzky and M. J. E. Golay, Anal. Chem., 1964, 36, 1627–1639 CrossRef CAS.
- M. Mofijur, H. H. Masjuki, M. A. Kalam, A. E. Atabani, I. M. R. Fattah and H. M. Mobarak, Ind. Crops Prod., 2014, 53, 78–84 CrossRef CAS PubMed.
- M. Mohibbe Azam, A. Waris and N. Nahar, Biomass Bioenergy, 2005, 29, 293–302 CrossRef CAS PubMed.
- A. Datta and B. K. Mandal, ASME 2013 International Mechanical Engineering Congress and Exposition, 2013.
- M. M. E.-G. El-Hagar, Int. J. Appl. Sci. Eng. Res., 2013, 2, 562–568 CAS.
- N.-O. Nylund, P. Aakko-Saksa and K. Sipilä, Status and outlook for biofuels, other alternative fuels and new vehicles, VTT, 2008 Search PubMed.
- M. I. Arbab, H. H. Masjuki, M. Varman, M. A. Kalam, S. Imtenan and H. Sajjad, Renewable Sustainable Energy Rev., 2013, 22, 133–147 CrossRef CAS PubMed.
- M. Arbab, H. Masjuki, M. Varman, M. A. Kalam, H. Sajjad and S. Imtenan, RSC Adv., 2014, 4, 37122–37129 RSC.
- A. N. Ozsezen and M. Canakci, Energy Convers. Manage., 2011, 52, 108–116 CrossRef CAS PubMed.
- S. Imtenan, H. H. Masjuki, M. Varman, M. A. Kalam, M. I. Arbab, H. Sajjad and S. M. Ashrafur Rahman, Energy Convers. Manage., 2014, 83, 149–158 CrossRef CAS PubMed.
- A. N. Ozsezen, M. Canakci, A. Turkcan and C. Sayin, Fuel, 2009, 88, 629–636 CrossRef CAS PubMed.
- S. Palash, M. Kalam, H. Masjuki, M. Arbab, B. Masum and A. Sanjid, Energy Convers. Manage., 2014, 77, 577–585 CrossRef CAS PubMed.
- H. Yongcheng, Z. Longbao, W. Shangxue and L. Shenghua, Proc. Inst. Mech. Eng., Part D, 2006, 220, 827–835 CrossRef.
- A. N. Ozsezen, M. Canakci and C. Sayin, Energy Fuels, 2008, 22, 1297–1305 CrossRef CAS.
- J. Li, L. Zhou, K. Pan, D. Jiang and J.-O. Chae, Evaluation of the thermodynamic process of indirect injection diesel engines by the first and second law, SAE Tech. Pap., 1995, 952055, DOI:10.4271/952055.
- J. B. Heywood, Internal combustion engine fundamentals, McGraw-Hill, New York, 2002 Search PubMed.
- A. Abu-Jrai, A. Tsolakis, K. Theinnoi, R. Cracknell, A. Megaritis, M. L. Wyszynski and S. E. Golunski, Energy Fuels, 2006, 20, 2377–2384 CrossRef CAS.
- K. Yehliu, A. L. Boehman and O. Armas, Fuel, 2010, 89, 423–437 CrossRef CAS PubMed.
- M. Lapuerta, O. Armas and J. Rodriguez-Fernandez, Prog. Energy Combust. Sci., 2008, 34, 198–223 CrossRef CAS PubMed.
- J. B. Hirkude and A. S. Padalkar, Appl. Energy, 2012, 90, 68–72 CrossRef CAS PubMed.
- E. Cecrle, C. Depcik, A. Duncan, J. Guo, M. Mangus, E. Peltier, S. Stagg-Williams and Y. Zhong, Energy Fuels, 2012, 26, 2331–2341 CrossRef CAS.
- Y. Di, C. Cheung and Z. Huang, Sci. Total Environ., 2009, 407, 835–846 CrossRef CAS PubMed.
- H. Wang, H. Hao, X. Li, K. Zhang and M. Ouyang, Appl. Energy, 2009, 86, 2257–2261 CrossRef CAS PubMed.
- I. M. Rizwanul Fattah, M. A. Kalam, H. H. Masjuki and M. A. Wakil, RSC Adv., 2014, 4, 17787–17796 RSC.
- L. Xinling and H. Zhen, Sci. Total Environ., 2009, 407, 2234–2244 CrossRef PubMed.
- S. K. Hoekman and C. Robbins, Fuel Process. Technol., 2012, 96, 237–249 CrossRef CAS PubMed.
- G. Knothe, Fuel Process. Technol., 2005, 86, 1059–1070 CrossRef CAS PubMed.
- D. Rakopoulos, Fuel, 2013, 105, 603–613 CrossRef CAS PubMed.
- S. Imtenan, M. Varman, H. H. Masjuki, M. A. Kalam, H. Sajjad, M. I. Arbab and I. M. Rizwanul Fattah, Energy Convers. Manage., 2014, 80, 329–356 CrossRef CAS PubMed.
- H. Sajjad, H. Masjuki, M. Varman, M. Kalam, M. Arbab, S. Imtenan and M. Rashed, RSC Adv., 2014, 4, 44529–44536 RSC.
- S. Hossain, H. Masjuki, M. Varman, M. A. Kalam and S. A. Rahman, RSC Adv., 2015, 5, 13068–13077 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra15548c |
| This journal is © The Royal Society of Chemistry 2015 |