Selective fluorescence sensing of salicylic acids using a simple pyrenesulfonamide receptor†
Ashwani Kumara,
Manik Kumer Ghoshb,
Cheol-Ho Choib and
Hong-Seok Kim*a
aSchool of Applied Chemical Engineering, Department of Applied Chemistry, Kyungpook National University, Daegu 702-701, Republic of Korea. E-mail: kimhs@knu.ac.kr; Fax: +82 53 9506594; Tel: +82 53 9505588
bDepartment of Chemistry and Green-Nano Materials Research Center, College of Natural Sciences, Kyungpook National University, Daegu 702-701, Republic of Korea
First published on 25th February 2015
Abstract
Pyrenesulfonamide and pyreneamide probes (2–5) were synthesized, and they were used in the sensing of salicylic acid derivatives. The ability of 3 to sense various salicylic acid derivatives was examined by UV-visible, fluorescence, and NMR spectroscopy; it was further supported by DFT calculations. The sensing of salicylic acid derivatives resulted in a significant quenching of the pyrene monomer emission. Among the tested salicylic acid derivatives, probe 3 exhibited the highest binding constant with 3,5-dinitrosalicylic acid (Ka = 2.65 × 105 M−1, measured at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in EtOH).
1 molar ratio in EtOH).
1. Introduction
Because of the significant roles that carboxylic acids play in metabolism/biology, there is a growing interest in being able to sense them.1 Carboxylic acids find several applications in various fields, such as the food industry,2 medical diagnosis,3 environmental monitoring,4 and process control.5 A variety of probes with different binding sites, such as amines (having N–H groups as hydrogen-bonding sites),6 quaternary ammonium/imidazolium salts (having positive charges for ionic interactions in addition to hydrogen bonding),7 ureas,8 amides,9 α-aminopyridines/α-amidopyridines10 α,α′-diaminopyridines/α,α′-diamidopyridines, and diamides11 (having two hydrogen-bonding sites in the same probe), linked to different fluorophores/heterocycles, have been used for the sensing of carboxylic acids.12 We focused our research efforts on the sensing of aromatic (instead of aliphatic) carboxylic acids.13In the last two decades, sulfonamides appended with various chromophores/fluorophores have found potential applications in the sensing of different analytes, such as metal ions, anions and neutral molecules.14 Recently, among different fluorophores, pyrenesulfonamides conjugated with various amino acids have been used for metal-ion sensing,15 and glucono-linked pyrenesulfonamides were found to behave as gelators in tetrahydrofuran–water mixtures.16 One of the butterfly-shaped cholesterol-conjugated pyrenesulfonamide dipodes exhibits ratiometric excimer emission: an increase in the concentration of water in solvents such as ethanol, methanol, and acetonitrile leads to a decrease in monomer emission.17
Recently, pyrene-appended aminopropylimidazole probe 1 was reported to be an effective sensor for salicylic acid.13b Probe 1, having an imidazole ring and an amino group (N–H) as hydrogen-bonding motifs and a pyrene fluorophore for the π–π interactions, was designed based on hydrogen-bonding complementarity between the host and guest and on an increase in the intensities of monomer emission bands. Because of the methylene (CH2) unit between the amino group and the pyrene unit, the lone pair of the amino nitrogen is not conjugated with the aromatic core of the pyrene unit, which results in a weak emission intensity of probe 1. Upon the addition of various aromatic carboxylic acids (ACAs) to a solution of probe 1 (3 μM in EtOH), a selective “switch on” of the fluorescence emission intensity of the pyrene monomer takes place. This occurs with salicylic acid (SA) and SA derivatives that have electron-withdrawing groups, such as 5-nitrosalicylic acid (5-NSA) and 5-iodosalicylic acid (5-ISA). It is caused by the restricted rotation of the benzene ring in SA and its derivatives, because of intramolecular hydrogen bonding between the phenolic O–H group and the neighboring C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group (Fig. 1).13b The degree of emission enhancement is dependent on the substituent and on the orientation of the benzoic acid (BA) hydroxyl group.
O group (Fig. 1).13b The degree of emission enhancement is dependent on the substituent and on the orientation of the benzoic acid (BA) hydroxyl group.
 | ||
| Fig. 1 Free rotation of the carboxylic acid group is possible in BA, free rotation of the carboxylic acid group is prohibited in SA, and scheme of complexation of probe 1 with SA. | ||
In the case of imidazole receptors, the imidazole C2–H group participates in hydrogen bonding with anions.18 Keeping in mind the active roles of the sulfonamide N–H and imidazole C2–H groups and the good fluorescence monomer/excimer emission of pyrene, we designed very simple pyrenesulfonamide/pyreneamide probes 2–5 for the detection of SA derivatives (Fig. 2). This is the first report in which pyrenesulfonamides are used for the sensing of SA derivatives.
2. Results and discussion
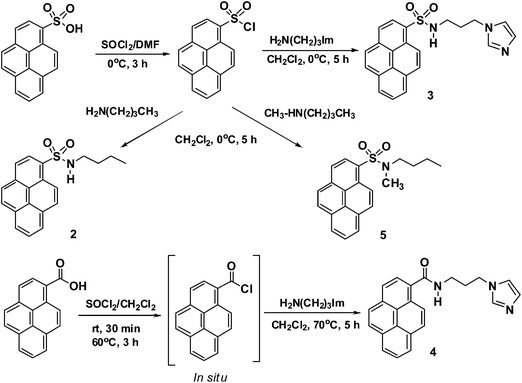
Probes 2–5 are easily synthesized in good yields, in a single step, by the reaction of either pyrenesulfonyl chloride or 1-pyrenecarboxylic acid chloride with amines (Scheme 1). The structures of 2–5 were confirmed by 1H and 13C NMR spectroscopy and by FAB mass spectrometry (see ESI†). The 1H NMR spectrum of 2 in CDCl3 shows four signals due to aliphatic protons, at δ 0.73 (triplet for CH3), δ 1.17–1.22 (multiplet for CH2), δ 1.35–1.39 (multiplet for CH2), and δ 2.94 (triplet for CH2), and a broad singlet at δ 4.73 for the N–H group, in addition to other signals due to aromatic protons. The mass spectrum of 2 clearly shows a molecular ion peak at m/z 337.1141 (M+). Similarly, the 1H NMR spectrum of probe 3 in DMSO-d6 shows three signals for the propyl CH2 groups (two multiplets at δ 1.68–1.72 and δ 2.73–2.75, one triplet at δ 3.84) and three singlets for the imidazole protons (at δ 6.72, δ 6.85, and δ 7.36 for H–b, H–c, and H–a, respectively). The 13C NMR spectrum of probe 3 shows a signal for the imidazole C-2 at δ 137.4. The mass spectrum of probe 3 clearly shows a molecular ion peak at m/z 390.1273 ([M + H]+). The 13C NMR spectrum of 4 clearly shows a peak due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group at δ 169.41. 1H and 13C NMR spectra of 5 show singlets at δ 2.80 and δ 34.54, respectively, for the NCH3 group. These spectral features indicate the successful formation of these probes.
O group at δ 169.41. 1H and 13C NMR spectra of 5 show singlets at δ 2.80 and δ 34.54, respectively, for the NCH3 group. These spectral features indicate the successful formation of these probes.
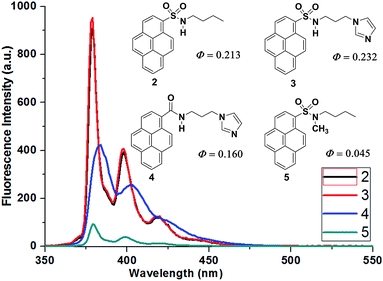
The UV-visible absorption spectrum of probe 2 exhibits typical pyrene absorption maxima at λmax = 322, 336, and 350 nm in EtOH. Upon excitation of 2 (1 μM in EtOH) at 336 nm, it exhibits fluorescence emission maxima at 379, 398, and 420 nm with a high quantum yield (Φ = 0.213); this stands in contrast to probe 1, which has a weak fluorescence emission. In case of probe 2, the extended conjugation between the sulfonamide nitrogen and the pyrene unit leads to easy charge transfer and a high quantum yield. Probe 3 has the highest quantum yield (Φ = 0.232) among these probes. Probe 4 also has a good quantum yield (Φ = 0.160), whereas probe 5 (having a NCH3 instead of a NH functionality in the sulfonamide) has the lowest quantum yield (Φ = 0.045); this clearly signifies the role of the sulfonamide NH functionality (Fig. 3). Upon the addition of 10 equiv. of an ACA to a solution of probe 2, the fluorescence emission intensity of 2 is quenched selectively with salicylic acid derivatives. The extent of quenching is >95% with 3,5-DNSA, 50% with 5-NSA, and 20% with 5-ISA, while other ACAs do not show any significant changes in the emission intensity of probe 2 (Fig. 4).
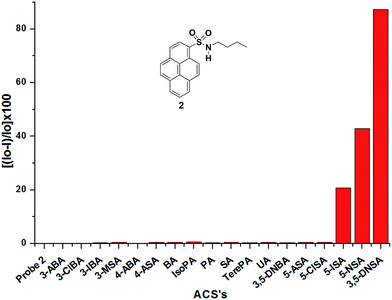 | ||
| Fig. 4 Bar diagram of the relative fluorescence intensities of probe 2 (1 μM in EtOH, λex = 336 nm, λem = 379 nm) in the presence of various ACAs. | ||
The degree of quenching of the fluorescence emission intensities of the probes is dependent on the substituent and on the orientation of the hydroxyl group of benzoic acid (BA). The 2-hydroxyl group of SA forms an intramolecular hydrogen bond with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, which restricts the rotation of the carboxylic acid group. The combined effects of the intermolecular hydrogen bonding ((a) S
O group, which restricts the rotation of the carboxylic acid group. The combined effects of the intermolecular hydrogen bonding ((a) S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–O, (b) SO2N–H⋯O
O⋯H–O, (b) SO2N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) and the intramolecular hydrogen bonding ((c) O–H⋯O
C) and the intramolecular hydrogen bonding ((c) O–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) between probe 2 and SA (or SA derivatives) bring two aromatic moieties close enough to each other to affect the fluorescence emission intensity of probe 2. The degree of the decrease in the fluorescence monomer emission intensity of probe 2 clearly shows that 3,5-DNSA interacts more strongly with probe 2 than 5-NSA (which, in turn, interacts more strongly than 5-ISA). This demonstrates that the degree of quenching of the fluorescence emission intensity depends on the electron-withdrawing nature of the aromatic substituents in SA derivatives. We attribute this to the fact that the aromatic rings of these SA derivatives are electron deficient; they can accept electron charge from the pyrenesulfonamide probe 2. In the absence of these SA derivatives, probe 2 is highly fluorescent because no charge transfer will occur, whereas in the presence of 3,5-DNSA, 5-NSA, or 5-ISA, the charge transfer occurs easily, resulting in the quenching of the fluorescence emission intensity.
C) between probe 2 and SA (or SA derivatives) bring two aromatic moieties close enough to each other to affect the fluorescence emission intensity of probe 2. The degree of the decrease in the fluorescence monomer emission intensity of probe 2 clearly shows that 3,5-DNSA interacts more strongly with probe 2 than 5-NSA (which, in turn, interacts more strongly than 5-ISA). This demonstrates that the degree of quenching of the fluorescence emission intensity depends on the electron-withdrawing nature of the aromatic substituents in SA derivatives. We attribute this to the fact that the aromatic rings of these SA derivatives are electron deficient; they can accept electron charge from the pyrenesulfonamide probe 2. In the absence of these SA derivatives, probe 2 is highly fluorescent because no charge transfer will occur, whereas in the presence of 3,5-DNSA, 5-NSA, or 5-ISA, the charge transfer occurs easily, resulting in the quenching of the fluorescence emission intensity.
Although molecules similar to salicylic acid (such as salicylaldehyde, salicylamide, salicylhydroxamic acid, anthranilic acid, picolinic acid, and indole-2-carboxylic acid (Fig. SI-1†) were also tested with probe 2, they did not cause any changes in the emission intensity of probe 2. This observation indicates that an α-phenolic OH is essential for the restriction of the rotation of the C(O)OH group. Electron-withdrawing groups such as nitro and iodo groups make the aromatic ring more electron deficient, so that the C(O)OH hydroxyl groups in 3,5-DNSA, 5-NSA, and 5-ISA act as hydrogen donors (hydrogen-bonding units) toward the sulfonamide oxygen. The C(O)OH carbonyl oxygens act as hydrogen acceptors (hydrogen-bonding units) toward the sulfonamide N–H functionality of probe 2. The formation of intermolecular hydrogen bonds is not very effective when picolinic acid, indole-2-carboxylic acid, anthranilic acid, salicylaldehyde, salicylamide, and salicylhydroxamic acid are used.
To explore the binding constants for the binding of probe 2 with salicylic acid derivatives, we carried out fluorescence titrations on probe 2. Upon the gradual addition of 3,5-DNSA to a solution of probe 2, the fluorescence emission intensity decreases until the emission is eventually quenched completely (Fig. SI 2 and SI-3†). The fluorescence titration shows that a 1:1 complex between probe 2 and 3,5-DNSA is formed with a high association constant (Ka = 2.83 × 104 M−1; see Table 1). Similarly, fluorescence titrations of probe 2 with 5-NSA and 5-ISA show the formation of 1:1 complexes as well, with association constants of 9.07 × 103 M−1 and 5.32 × 103 M−1, respectively, along with quenching of fluorescence emission intensity. The association constants for the complexation of 5-NSA and 5-ISA to probe 2 are approximately ten times lower than those measured for probe 1. On the other hand, the “switch off” of the fluorescence emission observed for probe 2 is the opposite of the “switch on” phenomenon observed for probe 1 (upon complexation with 5-NSA and 5-ISA).
To investigate the effect of the C2–H group of an imidazole ring, we designed and synthesized probe 3, having acidic sulfonamide N–H functionality for hydrogen bonding and C2–H of an imidazole ring as an additional hydrogen bonding motif. Upon excitation of probe 3 (1 μM in EtOH) at 336 nm, the emission spectrum shows strong pyrene monomer fluorescence emission peaks at λmax = 379, 398, and 420 nm with a high quantum yield (Φ = 0.232). Upon the addition of ACAs to the solution of probe 3, it exhibits behavior similar to probe 2, i.e., the degree of quenching of the pyrene monomer emission is 3,5-DNSA ≫ 5-NSA > 5-ISA (Fig. SI-4†). Upon the gradual addition of 3,5-DNSA to a solution of probe 3, the fluorescence emission intensity decreases with each addition and the emission is eventually quenched completely (Fig. 5). The fluorescence titration shows the formation of a 1:1 complex between 3 and 3,5-DNSA with a high association constant (Ka = 2.66 × 105 M−1).
Fluorescence titration experiments of probe 3 with the acids 3,5-DNSA, 5-NSA, and 5-ISA evidence the formation of 1:1 complexes between probe 3 and the acids, as well as quenching of the pyrene monomer emission intensity. The association constants are listed in Table 1.
Table 1 clearly shows that the imidazole ring in probe 3 results in association constants that are approximately 10 times higher than those measured for probe 2 (and they are similar to those measured for probe 1). This is probably due to hydrogen bonding of the imidazole C2–H hydrogen with more electronegative oxygens of SA derivatives.
To check, the sulfonamide N–H functionality is more active or less active than the amide N–H functionality with regard to the sensing of SA derivatives, we synthesized probe 4 (which has a C(O)NH group instead of a S(O)2NH group (probe 3)). Upon excitation of probe 4 (1 μM in EtOH) at 336 nm, its emission spectrum exhibits monomer fluorescence emission peaks at λmax = 383 and 402 nm, with a good quantum yield (Φ = 0.160). Upon the addition of ACAs to a solution of probe 4, it exhibits behavior that is similar to that of probe 3, i.e., quenching of the pyrene monomer emission intensity in the order 3,5-DNSA > 5-NSA. However, the degree of quenching of the fluorescence emission intensity is lower (Fig. SI-5†). Titration experiments of probe 4 with 3,5-DNSA and 5-NSA evidence the formation of 1:1 complexes between probe 4 and the acids, as well as quenching of the monomer fluorescence emission intensity. The association constants are listed in Table 1.
To understand the role of the sulfonamide N–H functionality, we synthesized probe 5, which has no N–H and imidazole C2–H functionalities. Upon excitation of probe 5 (1 μM in EtOH) at 336 nm, its emission spectrum exhibits very weak monomer fluorescence emission peaks at λmax = 379, 398, and 420 nm, with a low quantum yield (Φ = 0.045). The quantum yield of 5 is approximately 5 times smaller than that of 2, which has sulfonamide N–H functionality (Fig. 3); this clearly shows the significance of the N–H functionality. Upon the addition of ACAs to the solution of probe 5, it exhibits a 30% decrease in its emission intensity with 3,5-DNSA and a 10% decrease with 5-NSA; other ACAs did not significantly affect the fluorescence intensity of 5 (Fig. SI-6†). Titration experiments of probe 5 with 3,5-DNSA and 5-NSA evidence the formation of 1:1 complexes between probe 5 and these acids. The association constants, which are rather low, are listed in Table 1.
The studies of the binding of probes 2–5 to ACAs, using the changes in fluorescence, clearly show that probe 5 has a very weak monomer emission and a low quantum yield in comparison with probes 2–4 (Fig. 3), and that 5 undergoes very small changes in the intensity of fluorescence emission upon binding with 3,5-DNSA and 5-NSA (Fig. 6); the association constants are low (Table 1). The change in monomer fluorescence emission intensity of probe 5, when complexed with 3,5-DNSA, is probably due to hydrogen bonding of the 3,5-DNSA C(O)OH hydroxyl group with the sulfonamide oxygen of probe 5.
To further investigate the nature of the interaction and the mode of complexation of SA derivatives with probes 2–5, we carried out 1H NMR titrations of 3, 4, and 5 with SA and its derivatives.
The addition of 1 equiv. of 3,5-DNSA to a solution of probe 3 in DMSO-d6 leads to a significant downfield shift of the imidazole C2–H resonance (labeled a in Fig. 7) from δ 7.36 to δ 9.01. Signals due to protons b, c, d, e, and f shift downfield from δ 6.72 to δ 7.63, from δ 6.85 to δ 7.63, from δ 2.73 to δ 2.77, from δ 1.69 to δ 1.89, and from δ 3.83 to δ 4.16, respectively. Additionally, signals due to protons g and h shift upfield from δ 8.97 to δ 8.94 and from δ 8.55 to δ 8.54, respectively. The addition of 1 more equiv. of 3,5-DNSA to this solution does not result in further shifting of the proton signals, which clearly indicates that a 1:1 complex between probe 3 and 3,5-DNSA is formed (Fig. 7). The large changes in the chemical shifts of the labeled protons confirm that both the imidazole C2–H (proton a) and the sulfonamide N–H proton (which undergoes exchange with the deuteriums of DMSO-d6) are involved in hydrogen bonding with 3,5-DNSA. For this reason, the signals due to the aliphatic protons f and e are shifted more downfield than the signal due to protons d (see Table 2). The upfield shifting of the protons g and h clearly signifies that the sulfonamide oxygen is also involved in hydrogen bonding.
 | ||
| Fig. 7 Partial 1H NMR spectra (DMSO-d6) of (i) probe 3, (ii) 3 + 1 equiv. 3,5-DNSA, (iii) 3 + 2 equiv. 3,5-DNSA, and (iv) 3,5-DNSA. | ||
| Probes | 3 | 4 | 5 | ||||
|---|---|---|---|---|---|---|---|
| SA | 5-ISA | 5-NSA | 3,5-DNSA | 3,5-DNSAb | 3,5-DNSA | 3,5-DNSA | |
| a Positive values indicate downfield shifting and negative values indicate upfield shifting of the protons in the 1H NMR spectra upon complexation.b Measured in CD3OD. | |||||||
| a | 0.873 | 1.467 | 1.653 | 1.653 | 1.500 | 1.481 | NA |
| b | 0.480 | 0.810 | 0.910 | 0.910 | 0.725 | 0.819 | NA |
| c | 0.430 | 0.710 | 0.780 | 0.780 | 0.713 | 0.589 | NA |
| d | 0.023 | 0.038 | 0.038 | 0.042 | 0.022 | 0.071 | |
| e | 0.110 | 0.181 | 0.201 | 0.204 | 0.226 | 0.130 | |
| f | 0.175 | 0.280 | 0.328 | 0.330 | 0.410 | 0.239 | |
| g | −0.012 | −0.024 | −0.028 | −0.035 | −0.047 | 0.040 | −0.001 |
| h | −0.003 | −0.004 | −0.006 | −0.010 | −0.03 | 0.020 | −0.001 |
We also performed the 1H NMR titration of probe 3 with 3,5-DNSA in CD3OD; in this solvent, the signals display shifts similar to those observed in DMSO-d6 (data obtained in CD3OD are listed in Table 2). Because of the good solubility of probe 3 in DMSO-d6, we carried out the titrations with 5-NSA, 5-ISA, and SA in DMSO-d6; the measured chemical shift differences are listed in Table 2 (Fig. SI-7–SI-12†). The binding behaviors of 5-NSA and 3,5-DNSA were found to be the same.
From Table 2 it is clear that 5-ISA causes smaller chemical shift differences than 5-NSA, but larger chemical shift differences than SA. This implies that the strong electron-withdrawing nitro group (NO2) in 3,5-DNSA and 5-NSA causes these SA derivatives to bind more strongly than 5-ISA (which, in turn, binds more strongly than SA). Hence, the electron-withdrawing group controls the behaviors of the SA derivatives with regard to binding to probe 3. In the 1H NMR titration experiments of probe 3 with 5-NSA, 5-ISA, and SA, we found that the proton signals of these acids (i.e., protons w, x, y, and z) shift upfield when the acids interact with probe 3 (Figs. SI-7, SI-9 and SI-11†). This indicates that these acids accept electron charge from the electron-rich pyrene ring of probe 3 through charge transfer. This is also supported by the decay time of probe 3 and its complex with 3,5-DNSA that shows only slight difference in the decay time as compared with 3,5-DNBA (Fig. SI-13 and SI-14†). So the formation of [3·3,5-DNSA]-complex occurs in the ground state that overrules the possibility of PET phenomenon. With regard to probe 4, the addition of 1 equiv. of 3,5-DNSA causes downfield shifting of all of the proton signals (a–h); the addition of one extra equiv. of 3,5-DNSA does not cause any further downfield shifting (Fig. SI-15 and SI-16†). This confirms the formation of a 1:1 complex between probe 4 and 3,5-DNSA. Hence, 3,5-DNSA probably binds probe 3 in the same way that it binds probe 4. The addition of 1 equiv. of 3,5-DNSA to a DMSO-d6 solution of probe 5 leaves most of the proton signals unaffected, although protons g and h of the pyrene ring do show some upfield shifting (Fig. SI-17 and SI-18†). This could be due to hydrogen bonding of the salicylic acid, i.e. OH of the COOH with sulfonamide oxygen.
To understand the mechanism of the interaction of SA derivatives with probe 3, we performed energy-minimization calculations using gradient-correlated density functional theory (DFT). The optimized structures of the complexes 3·3,5-DNSA and 3·5-NSA are presented in Fig. 8. The binding energies of 5-NSA and 3,5-DNSA with probe 3 are 7.3 and 14.3 kcal mol−1, respectively, showing that probe 3 has a higher tendency to bind 3,5-DNSA than to bind 5-NSA. This agrees with our experiments, in which we found that 3,5-DNSA has the higher association constant (Table 1). The complex 3·3,5-DNSA is 7.0 kcal mol−1 more stable than the complex 3·5-NSA, which is due to the additional strong hydrogen bond between the OH and NO2 groups in 3,5-DNSA.
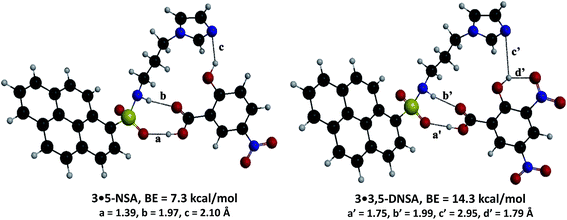 | ||
| Fig. 8 Energy-minimized geometries of complexes of probe 3 with 5-NSA and 3,5-DNSA, calculated by B3LYP/6-31G*. | ||
The electronic transitions are predominantly characterized by HOMO → LUMO at the excited-state geometry. We find that binding of 5-NSA and 3,5-DNSA to 3 results in a decrease of the HOMO–LUMO energy difference; the decrease is more pronounced with 3,5-DNSA than with 5-NSA, which leads to strong binding between 3,5-DNSA and probe 3 (Fig. 9, SI-19 and SI-20, and Table SI-1†). Using B3LYP/6-31G*, the fluorescence emission spectra of probe 3 and its complexes with 5-NSA and 3,5-DNSA were obtained (they are shown in Fig. SI-21†). The calculated spectra also support the experiment results, i.e., the quenching of fluorescence intensity of probe 3 is higher with 3,5-DNSA than with 5-NSA, which leads to a higher association constant. We have also found that probe 3 can be used for the detection of 3,5-DNSA over a wide pH range between 3–12 (Fig. SI 22†).
 | ||
| Fig. 9 Calculated (B3LYP/6-31G*) molecular orbitals of 5-NSA, 3,5-DNSA, probe 3, and complexes of probe 3 with 5-NSA and 3,5-DNSA. | ||
3. Conclusions
In conclusion, this is the first report in which simple pyrenesulfonamides have been used for the recognition of SA derivatives. Probes 2 and 3 both have a sulfonamide N–H functionality as a hydrogen bonding motif (which plays a crucial role), and the presence of the C2–H group in the imidazole ring in probe 3 provides the additional hydrogen bonding site that holds the SA in close proximity to the pyrene ring. This results in a high association constant for 3,5-DNSA and in complete quenching of the fluorescence emission intensity due to charge transfer from the pyrene unit to the electron-deficient aromatic ring of 3,5-DNSA. 1H NMR titration experiments and DFT calculations clearly support the mechanism with which the probes interact with SA and SA derivatives.4. Experimental section
4.1. General
Melting points were determined using a Thomas-Hoover capillary melting point apparatus and are uncorrected. 1H and 13C NMR spectra were recorded on a Bruker AM-400 spectrometer, using Me4Si as the internal standard. FAB mass spectrometry was performed at the KBSI Daegu branch. UV-visible absorption spectra were recorded on a Shimadzu UV-1650PC spectrophotometer. Fluorescence spectra were measured on a Shimadzu RF-5301 fluorescence spectrometer equipped with a xenon discharge lamp, 1 cm quartz cells and with slit width 3 nm. The fitting of the fluorescence titration data was done using gunplot 4.6 software. All of the measurements were performed at 298 K. Analytical grade ethanol was purchased from Merck. All other materials for syntheses were purchased from Aldrich Chemical Co. and they were used as received. Quantum yields (Φ) were determined using the procedure reported in the literature.194.2. Theoretical calculations
The geometries of all compounds were optimized using gradient-correlated density functional theory (DFT) using the Becke three-parameter exchange functional20 and the Lee–Yang–Parr correlation functional (B3LYP).21 All-electron 6-31G(d, p) basis sets were used. Time-dependent density functional theory (TD-DFT) was used for excited-state calculations. The fluorescence emission spectra were plotted using excitation and oscillator strength of the molecule in TD-DFT calculations. TD-B3LYP/6-31G(d, p) calculations were used for excited-state optimizations of 5-NSA, 3,5-DNSA, probe 3, and for SA complexes of 3. All of the calculations herein were performed with GAMESS (General Atomic and Molecular Electronic Structure System).224.3. Syntheses of probes 2–5
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent (Rf = 0.15), to give 3 as a light yellow solid (344 mg, 71% yield). Melting point: 200 °C (CH2Cl2/hexane). 1H NMR (400 MHz, DMSO-d6) δ 1.68–1.72 (m, 2H, CH2), 2.73–2.75 (m, 2H, CH2), 3.84 (t, J = 6.8 Hz, 2H, CH2), 6.72 (s, 1H, ArH), 6.85 (s, 1H, ArH), 7.36 (s, 1H, ArH), 8.23 (t, J = 7.6 Hz, 1H, ArH), 8.31 (d, J = 9.1 Hz, 1H, ArH), 8.41 (d, J = 8.8 Hz, 1H, ArH), 8.44 (d, J = 8.3 Hz, 1H, ArH), 8.48–8.51 (m, 3H, 3 × ArH), 8.57 (d, J = 8.1 Hz, 1H, ArH), 8.99 (d, J = 9.4 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6) δ 31.07 (CH2), 43.38 (CH2), 119.43, 123.54, 123.63, 124.64, 124.66, 127.14, 127.26, 127.46, 127.49, 127.57, 128.62, 129.97, 130.04, 130.39, 130.90, 132.52, 134.39, 137.40. HR-FAB mass calcd for C22H19N3O2S ([M + H]+): 390.1276; found: m/z 390.1273.
1) as the eluent (Rf = 0.15), to give 3 as a light yellow solid (344 mg, 71% yield). Melting point: 200 °C (CH2Cl2/hexane). 1H NMR (400 MHz, DMSO-d6) δ 1.68–1.72 (m, 2H, CH2), 2.73–2.75 (m, 2H, CH2), 3.84 (t, J = 6.8 Hz, 2H, CH2), 6.72 (s, 1H, ArH), 6.85 (s, 1H, ArH), 7.36 (s, 1H, ArH), 8.23 (t, J = 7.6 Hz, 1H, ArH), 8.31 (d, J = 9.1 Hz, 1H, ArH), 8.41 (d, J = 8.8 Hz, 1H, ArH), 8.44 (d, J = 8.3 Hz, 1H, ArH), 8.48–8.51 (m, 3H, 3 × ArH), 8.57 (d, J = 8.1 Hz, 1H, ArH), 8.99 (d, J = 9.4 Hz, 1H, ArH). 13C NMR (100 MHz, DMSO-d6) δ 31.07 (CH2), 43.38 (CH2), 119.43, 123.54, 123.63, 124.64, 124.66, 127.14, 127.26, 127.46, 127.49, 127.57, 128.62, 129.97, 130.04, 130.39, 130.90, 132.52, 134.39, 137.40. HR-FAB mass calcd for C22H19N3O2S ([M + H]+): 390.1276; found: m/z 390.1273.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). HR-FAB mass calcd for C23H20N3O ([M + H]+): 354.1606; found: m/z 354.1610.
O). HR-FAB mass calcd for C23H20N3O ([M + H]+): 354.1606; found: m/z 354.1610.Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Science, ICT, and Future Planning (2013R1A1A2006777). H.Kim thanks professor Taiha Joo at POSTECH for valuable discussion and deacy time experiment.References
- (a) L. Styrer, Biochemistry, W. H. Freeman, New York, 3rd edn, pp. 188, 373–394, 376 and 575 Search PubMed; (b) M. Afran, H. R. Athar and M. Ashar, J. Plant Physiol., 2007, 164, 685 CrossRef PubMed.
- M. S. Maynor, T. L. Nelson, C. O'Sullivan and J. J. Lavigne, Org. Lett., 2007, 9, 3217 CrossRef CAS PubMed.
- A. T. Gates, S. O. Fakayode, M. Lowry, G. M. Ganea, A. Murugeshu, J. W. Robinson, R. M. Strongin and I. M. Warner, Langmuir, 2008, 24, 4107 CrossRef CAS PubMed.
- V. Adam, J. Zehnalek, J. Petrlova, D. Potesil, B. Sures, L. Trnkova, F. Jelen, J. Vitecek and R. Kizek, Sensors, 2005, 5, 70 CrossRef CAS PubMed.
- D. James, S. M. Scott, Z. Ali and W. T. O'Hare, Microchim. Acta, 2005, 149, 1 CrossRef CAS.
- (a) K. Ghosh, T. Sen and R. Frohlich, Tetrahedron Lett., 2007, 48, 7022 CrossRef CAS PubMed; (b) A. K. Mahapatra, P. Sahoo, S. Goswami and H.-K. Fun, J. Lumin., 2011, 131, 59 CrossRef PubMed; (c) M. Lee, H. Zali-Boeini, F. Li, L. F. Lindoy and K. A. Jolliffe, Tetrahedron, 2013, 69, 38 CrossRef CAS PubMed; (d) X. Yang, X. Liu, K. Shen, Y. Fu, M. Zhang, C. Zhu and Y. Cheng, Org. Biomol. Chem., 2011, 9, 6011 RSC.
- M. W. Ahmad, S. H. Kim and H.-S. Kim, Tetrahedron Lett., 2011, 52, 6743 CrossRef CAS PubMed.
- (a) S. Goswami, S. Jana, S. Dey, D. Sen, H.-K. Fun and S. Chantrapromma, Tetrahedron, 2008, 64, 6426 CrossRef CAS PubMed; (b) A. I. Oliva, L. Simon, F. M. Muniz, F. Sanz and J. R. Moran, Tetrahedron, 2004, 60, 3755 CrossRef CAS PubMed.
- (a) G. Moore, C. Papamicael, V. Levacher, J. Bourguignon and G. Dupas, Tetrahedron, 2004, 60, 4197 CrossRef CAS PubMed; (b) M. Almaraz, M. Martin, J. V. Hernandez, M. C. Caballero and J. R. Moran, Tetrahedron Lett., 1998, 39, 1811 CrossRef CAS.
- (a) S. J. Geih, C. Vicent, E. Fan and A. D. Hamilton, Angew. Chem., Int. Ed., 1993, 32, 119 CrossRef; (b) S. Goswami, K. Ghosh and M. Halder, Tetrahedron Lett., 1999, 40, 1735 CrossRef CAS; (c) S. Goswami, A. Hazra and H.-K. Fun, J. Inclusion Phenom. Macrocyclic Chem., 2010, 68, 461 CrossRef CAS; (d) S. Goswami, N. K. Das, D. Sen and H.-K. Fun, Supramol. Chem., 2010, 22, 532 CrossRef CAS; (e) K. Ghosh, G. Masanta, R. Frohlich, I. D. Petsalakis and G. Theodorakopoulos, J. Phys. Chem. B, 2009, 113, 7800 CrossRef CAS PubMed; (f) K. Ghosh, T. Sen and R. Frohlich, Tetrahedron Lett., 2007, 48, 2935 CrossRef CAS PubMed.
- (a) S. Goswami, N. K. Das, D. Sen, G. Hazra, J. H. Goh, Y. C. Sing and H.-K. Fun, New J. Chem., 2011, 35, 2811 RSC; (b) M. L. Mussons, C. Raposo, J. Anaya, M. Grande, J. R. Moran and M. C. Caballero, J. Chem. Soc., Perkin Trans. 1, 1992, 3125 RSC; (c) S. Goswami, K. Ghosh and S. Dasgupta, J. Org. Chem., 2000, 65, 1907 CrossRef CAS; (d) G. Moore, V. Levacher, J. Bourguignon and G. Dupas, Tetrahedron Lett., 2001, 42, 261 CrossRef CAS.
- (a) H.-C. Chou, C.-H. Hsu, Y.-M. Cheng, C.-C. Cheng, H.-W. Liu, S.-C. Pu and P.-T. Chou, J. Am. Chem. Soc., 2004, 126, 1650 CrossRef CAS PubMed; (b) K. Uzarevic, I. Halasz, I. Dilovic, N. Bregovic, M. Rubcic, D. Matkovic-C alogovic and V. Tomisic, Angew. Chem., Int. Ed., 2013, 52, 5504 CrossRef CAS PubMed; (c) K. Ghosh, I. Saha, G. Masanta, E. B. Wang and C. A. Parish, Tetrahedron Lett., 2010, 51, 343 CrossRef CAS PubMed; (d) S. Zakavi, M. N. Ragheb and M. Rafiee, Inorg. Chem. Commun., 2012, 22, 48 CrossRef CAS PubMed; (e) T. Kusukawa, K. Toyama, S. Takeshita and S. Tanaka, Tetrahedron, 2012, 68, 9973 CrossRef CAS PubMed; (f) P. Saliuga, N. Kaur, J. Kang, N. Singh and D. O. Jang, Tetrahedron, 2013, 69, 9001 CrossRef PubMed; (g) S. Goswami, K. Ghosh and R. Mukherjee, Tetrahedron, 2001, 57, 4987 CrossRef CAS; (h) T. Moriuchi, K. Yoshida and T. Hirao, Org. Lett., 2003, 5, 4285 CrossRef CAS PubMed; (i) S. Goswami, S. Dey, H.-K. Fun, S. Anjum and A.-U. Rahman, Tetrahedron Lett., 2005, 46, 7187 CrossRef CAS PubMed; (j) S. Goswami, S. Jana, S. Dey, I. A. Razak and H.-K. Fun, Supramol. Chem., 2006, 18, 571 CrossRef CAS; (k) S. Goswami, S. Jana and H.-K. Fun, CrystEngComm, 2008, 10, 507 RSC; (l) C. B. Aakeroy, J. Desper, B. Leonard and J. F. Urbina, Cryst. Growth Des., 2005, 5, 865 CrossRef; (m) J. R. Jadhav, M. W. Ahmad and H.-S. Kim, Tetrahedron Lett., 2010, 51, 5954 CrossRef CAS PubMed.
- (a) J. Yoon, J. R. Jadhav, J. M. Kim, M. Cheong, H.-S. Kim and J. Kim, Chem. Commun., 2014, 50, 7670 RSC; (b) M. W. Ahmad, B.-Y. Kim and H.-S. Kim, New J. Chem., 2014, 38, 1711 RSC.
- (a) D. Elbaum, S. K. Nair, M. W. Patchan, R. B. Thompson and D. W. Christianson, J. Am. Chem. Soc., 1996, 118, 8381 CrossRef CAS; (b) J. T. Suri, D. B. Cordes, F. E. Cappuccio, R. A. Wessling and B. Singaram, Angew. Chem., Int. Ed., 2003, 42, 5857 CrossRef CAS PubMed; (c) T. W. Kim, J.-H. Park and J.-I. Hong, J. Chem. Soc., Perkin Trans. 2, 2002, 923 RSC; (d) Y. M. Chung, B. Raman, D.-S. Kim and K. H. Ahn, Chem. Commun., 2006, 186 RSC; (e) M. T. Huggins, T. Butler, P. Barber and J. Hunt, Chem. Commun., 2009, 5254 RSC; (f) W. Jiang, Y. Cao, Y. Liu and W. Wang, Chem. Commun., 2010, 46, 1944 RSC; (g) C. N. Carroll, B. A. Coombs, S. P. McClintock, C. A. Johnson II, O. B. Berryman, D. W. Johnson and M. M. Haley, Chem. Commun., 2011, 47, 5539 RSC; (h) J.-M. Kim, C. R. Lohani, L. N. Neupane, Y. Choi and K.-H. Lee, Chem. Commun., 2012, 48, 3012 RSC; (i) H. Aboubakr, H. Brisset, O. Siri and J.-M. Raimundo, Anal. Chem., 2013, 85, 9968 CrossRef CAS PubMed; (j) N. Laurieri, M. H. J. Crawford, A. Kawamura, I. M. Westwood, J. Robinson, A. M. Fletcher, S. G. Davies, E. Sim and A. J. Russell, J. Am. Chem. Soc., 2010, 132, 3238 CrossRef CAS PubMed; (k) J. Yin, Y. Kwon, D. Kim, D. Lee, G. Kim, Y. Hu, J.-H. Ryu and J. Yoon, J. Am. Chem. Soc., 2014, 136, 5351 CrossRef CAS PubMed; (l) R. Sakai, E. B. Barasa, N. Sakai, S.-I. Sato, T. Satoh and T. Kakuchi, Macromolecules, 2012, 45, 8221 CrossRef CAS; (m) V. Luxami, A. S. Gupta and K. Paul, New J. Chem., 2014, 38, 2841 RSC; (n) N. V. Ghule, S. V. Bhosale and S. V. Bhosale, RSC Adv., 2014, 4, 27112 RSC; (o) Y. Cao, L. Ding, S. Wang, Y. Liu, J. Fan, W. Hu, P. Liu and Y. Fang, ACS Appl. Mater. Interfaces, 2014, 6, 49 CrossRef CAS PubMed; (p) T. Ema, K. Okuda, S. Watanabe, T. Yamasaki, T. Minami, N. A. Esipenko and P. Anzenbacher, Org. Lett., 2014, 16, 1302 CrossRef CAS PubMed; (q) A. Kumar and H.-S. Kim, New J. Chem., 2015 10.1039/c4nj01603c.
- (a) M.-H. Yang, P. Thirupathi and K.-H. Lee, Org. Lett., 2011, 13, 5028 CrossRef CAS PubMed; (b) L. N. Neupane, J.-Y. Park, J. H. Park and K.-H. Lee, Org. Lett., 2013, 15, 254 CrossRef CAS PubMed; (c) H. J. Kim, J. Hong, A. Hong, S. Ham, J. H. Lee and J. S. Kim, Org. Lett., 2008, 10, 1963 CrossRef CAS PubMed; (d) S. Jang, P. Thirupathi, L. N. Neupane, J. Seong, H. Lee, W. I. Lee and K.-H. Lee, Org. Lett., 2012, 14, 4746 CrossRef CAS PubMed.
- (a) N. Yan, Z. Xu, K. K. Diehn, S. R. Raghavan, Y. Fang and R. G. Weiss, J. Am. Chem. Soc., 2013, 135, 8989 CrossRef CAS PubMed; (b) N. Yan, Z. Xu, K. K. Diehn, S. R. Raghavan, Y. Fang and R. G. Weiss, Langmuir, 2013, 29, 793–805 CrossRef CAS PubMed.
- K. Zhao, T. Liu, G. Wang, X. Chang, D. Xue, K. D. Belfield and Y. Fang, J. Phys. Chem. B, 2013, 117, 5659–5667 CrossRef CAS PubMed.
- (a) J. Yoon, S. K. Kim, N. J. Singh and K. S. Kim, Chem. Soc. Rev., 2006, 35, 355 RSC; (b) S. Kumar, V. Luxami and A. Kumar, Org. Lett., 2008, 10, 5549 CrossRef CAS PubMed; (c) Z. Xu, S. K. Kim and J. Yoon, Chem. Soc. Rev., 2010, 39, 1457 RSC; (d) F. Zapata, A. Caballero, N. G. White, T. D. W. Claridge, P. J. Costa, V. Felix and P. D. Beer, J. Am. Chem. Soc., 2012, 134, 11533 CrossRef CAS PubMed; (e) E. Faggi, R. Porcar, M. Bolte, S. V. Luis, E. Garcia-Verdugo and I. Alfonso, J. Org. Chem., 2014, 79, 9141 CrossRef CAS PubMed; (f) J. Cai and J. L. Sessler, Chem. Soc. Rev., 2014, 43, 6198 RSC; (g) C. Gao, G. Gao, J. Lan and J. You, Chem. Commun., 2014, 50, 5623 RSC; (h) J. R. Jadhav, M. W. Ahmad and H.-S. Kim, Bull. Korean Chem. Soc., 2011, 32, 2933 CrossRef CAS.
- A. Kumar, V. Vanita, A. Walia and S. Kumar, Sens. Actuators, B, 2013, 177, 904 CrossRef CAS PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS PubMed.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter, 1988, 37, 785 CrossRef CAS.
- M. W. Schmidt, K. K. Balbridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. Su, T. L. Windus, M. Dupuis and J. A. J. Montgomery, J. Comput. Chem., 1993, 14, 1347 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra00565e |
| This journal is © The Royal Society of Chemistry 2015 |