Preparation, characterization and antifouling properties of polyacrylonitrile/polyurethane blend membranes for water purification†
Abstract
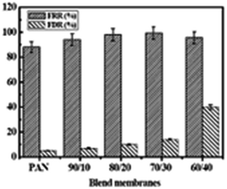
Casting of flat sheet polyacrylonitrile (PAN)/polyurethane (PU) blend membranes was reported for the first time in this work. PU makes the membrane more porous. Ternary phase diagram indicates that the addition of PU increases the thermodynamic instability of the blend. The average pore size of the membrane increased from 11 nm to 18 nm for pure PAN and the PAN/PU blend membranes. The membranes were characterized in terms of permeability, porosity, molecular weight cut-off (MWCO), contact angle, mechanical strength, scanning electron microscope (SEM), atomic force microscope (AFM), differential scanning calorimetry (DSC) and Fourier transform infrared spectroscopy (FTIR). The AFM images indicated that the surface roughness of the membrane increased with an increase in the concentration of PU. Addition of PU also imparted hydrophilicity to the membranes. FTIR and DSC measurements confirm the polymer compatibility of the PAN/PU blend. The PAN/PU blend 70/30 membrane exhibited the maximum antifouling characteristics with a 99% flux recovery ratio associated with the complete removal of turbidities and organic matter.

 Please wait while we load your content...
Please wait while we load your content...