Study of N-benzylidene derivatives synthesized as corrosion inhibitors for copper in HCl solution
Mehdi Shabani-Nooshabadi*a,
Mohsen Behpoura,
Faezeh Sadat Razavia,
Masood Hamadanianbc and
Vajihe Nejadshafieea
aDepartment of Analytical Chemistry, Faculty of Chemistry, University of Kashan, Kashan, P.O. Box 87317-51167, Iran. E-mail: m.shabani@kashanu.ac.ir; Fax: +98 3155912935; Tel: +98 3155912930
bInstitute of Nanosciences and Nanotechnology, University of Kashan, Kashan, Iran
cDepartment of Physical Chemistry, Faculty of Chemistry, University of Kashan, Kashan, Iran
First published on 23rd February 2015
Abstract
Corrosion inhibition of the two N-benzylidene derivative compounds, namely N-benzylidene pyridin – 2 amine (NBPA) and N-(4-choloromethylbenzylidene) pyridin – 2 amine (NCMBPA) as corrosion inhibitors for copper in hydrochloric acid 6.0 M have been studied by electrochemical methods such as potentiodynamic polarization measurements and electrochemical impedance spectroscopy. Potentiodynamic polarization results show that corrosion potentials shift to anodic regions in the presence of N-benzylidene compounds compared to the blank solution. The electrochemical measurements indicated that the inhibition efficiency for NCMBPA about 92% and NBPA about 85%. The adsorption of inhibitors on the copper surface follows from Langmuir adsorption isotherm. Activation energy (Ea), Gibbs free energy (ΔGads), enthalpy (ΔH*), and entropy (ΔS*) of corrosion process are determined by using the experimental measurements.
1. Introduction
Copper and its alloys are extensively applied in many fields on account of their electrical, thermal and mechanical properties such as ductility, hardness, strength and low price. Safety of copper is of exacting interest, particularly in acidic media, which also includes chloride ions.1–6Copper is susceptible against corrosion in such separate surroundings, although it is highly resistant in practically neutral or slightly alkaline aqueous environment. The utilization of suitable inhibitor to offer an explanation the particular corrosion mechanism of copper in acidic chloride environments is essential. It is commonly accepted that anodic dissolution of copper in chloride environments is influenced by the chloride concentration and the acidity of the medium. At chloride concentrations lower than 1.0 M, the dissolution of copper occurs through formation of CuCl which is not defensive enough and is changed to the soluble CuCl2− by reacting with excess chloride. Alternatively, at concentrations higher than 1.0 M, higher copper complexes such as CuCl32− and CuCl43−are formed, in addition to the ones with fewer chlorides, such as CuCl and CuCl2−.7
Most of acid corrosion inhibitors are organic compounds containing electronegative atoms (such as, N, S, P, O, etc.), the unsaturated bounds (such as, double bonds or triple bonds, etc.) and the plane conjugated systems containing all kinds of aromatic cycles.8–13 The inhibiting action of these compounds is usually attributed to their interactions with copper surface by their adsorption.14–16 The usage of nitrogen and sulfur containing organic compounds as corrosion inhibitor for copper has been commonly investigated.17–27 Some Schiff bases have been recently studied as corrosion inhibitors for different metals and alloys in acid media.28–32 These materials usually become effective by adsorption on the metal surface. The adsorbed species protect the metal from the aggressive medium, which causes decay of the metal. Adsorption depends on not only the nature and charge of the metal but also on the chemical structure of the inhibitor.
Increasing popularity of Schiff bases in the field of corrosion inhibition science based on the ease of synthesis from relatively inexpensive starting materials and their eco-friendly or low toxic properties.33,34 The high inhibitory performance of these compounds results from the substitution of different heteroatoms (N, O, Cl and Br) and π-electrons in their structure in besides the presence of imine (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) functional group.35,36 This organic compound having general formula R–C
N–) functional group.35,36 This organic compound having general formula R–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–R′ where R and R′ are aryl, alkyl or cycloalkyl or heterocyclic groups formed by the condensation of an amine and a carbonyl group, is a potential inhibitor. These molecules normally form a very thin and persistent adsorbed film that lead to decrease in the corrosion rate due to the slowing down of cathodic, anodic reaction or both.37–39 In order to improve the inhibitive force, synergistic inhibition effect is an effective method.40
N–R′ where R and R′ are aryl, alkyl or cycloalkyl or heterocyclic groups formed by the condensation of an amine and a carbonyl group, is a potential inhibitor. These molecules normally form a very thin and persistent adsorbed film that lead to decrease in the corrosion rate due to the slowing down of cathodic, anodic reaction or both.37–39 In order to improve the inhibitive force, synergistic inhibition effect is an effective method.40
In the present work, the inhibition effect of NBPA and NCMBPA on the corrosion of copper in 6.0 M HCl solution was firstly investigated by potentiodynamic polarization curves, electrochemical impedance spectroscopy (EIS). The adsorption isotherm and adsorption free energy were found and discussed. Effects of inhibitor concentration, temperature on the corrosion inhibition were fully investigated. Quantum chemical calculation of including the solvent effect is applied to study the difference in theoretical parameters between neutral inhibitor molecule and protonated inhibitor molecule. It is estimated to collect useful information on the inhibition effect of NBPA and NCMBPA on copper corrosion in HCl solution.
2. Experimental
2.1. Material
The metal of this study was copper having 99.9% purity. Copper panels were rectangular in size (1.0 cm, 1.0 cm, 0.1 cm). The panels were soldered with Cu-wire for electrical connection and mounted into the epoxy resin to offer only one active flat surface exposed to the corrosive environment. Prepare panels incorporated abrasion with SiC abrasive paper 400–2500 grades. The above samples were ultrasonically cleaned with ethanol and double distilled water, respectively. Then, they were dried at room temperature before immersion in experimental solution.
2.2. Electrochemical measurement
Electrochemical studies were carried out using an AUTOLAB model PGSTAT 30. The usual three electrode system was used for this purpose. Copper specimen was used as a working electrode. Pt electrode and silver–silver chloride Ag/AgCl (3.0 M Cl−) served as counter and reference electrodes, respectively. All polarization and impedance curves were recorded at room temperature (25 ± 2 °C). The working electrode was immersed in the test solution for 1000 seconds until a steady state open-circuit potential was attained. NOVA software was used for fitting impedance data in an equivalent circuit as well as for extrapolating Tafel slopes. Experiments were carried out in triplicate to ensure reproducibility of results.2.3. Electrochemical impedance spectroscopy study
The EIS measurements were performed over the frequency range 100 kHz to 100 mHz at the open circuit potential by superimposing alternating current (AC) signal of 0.01 V after immersion for 1000 second in the corrosive media. The inhibition efficiencies for each inhibitor concentration were calculated using the eqn (1):
 | (1) |
2.4. Potentiodynamic polarization
The potentiodynamic polarization curves were recorded from −200 to 200 mV (vs. Ag/AgCl (3.0 M Cl−)) corresponding to the corrosion potential with scan rate of 0.01 mV s−1. The inhibition efficiencies at different inhibitor concentrations were estimated using the eqn (2):
 | (2) |
2.5. Quantum chemical study
All the calculations were performed with of complete geometry optimization by using the standard Gaussian 98 software package with semi-empirical methods including the Austin Model B3LYP with 6-311G** basis set and Conductor-like Screening Model' or COSMO method which includes PCM and CPCM methods.42 COSMO is a calculation method for determining the electrostatic interaction of a molecule with a solvent. The polarizable continuum model (PCM) is a commonly used method in computational chemistry to model solvation effects. If it were necessary to consider each solvent molecule as a separate molecule, the computational cost of modeling a solvent-mediated chemical reaction would grow prohibitively high. Modeling the solvent as a polarizable continuum, rather than individual molecules, makes initio computation feasible. Two types of PCMs have been popularly used: dielectric PCM (D-PCM) which deals the continuum as a polarizable dielectrics and conductor-like PCM (C-PCM) which deals the continuum as a conductor-like picture similar to COSMO Solvation Model.69,70 The molecule sketch of Schiff bases was drawn in Gauss View 3.0.2.6. Scanning electron microscopy (SEM) studies
In order to get insight into the changes on surface of corrosive samples before and after the addition of inhibitors, the specimens were first immersed in 6.0 M HCl in the absence and presence of 0.01 M NBPA or NCMBPA for 3 h, respectively. Then taken out from the test solutions, cleaned with double-distilled water and dried at room temperature. The SEM images were conducted using a Philips scanning electronic microscope model XL30.3. Result and discussion
3.1. Electrochemical impedance spectroscopy measurements
Fig. 2 shows the Nyquist plots of copper in a 6.0 M HCl solution in the absence and presence of NBPA and NCMBPA in different concentrations. As seen in Fig. 2. The Nyquist plots of blank solutions (hydrochloric acid 6.0 M) show Warburg impedance at low frequencies (Fig. 3) and only one depressed capacitive loop. Warburg impedance in the absence of inhibitors shows that the dissolution mechanism of copper is controlled by mass transport rate. The diffusion step of copper dissolution in several papers is ascribed to the transport of chloride ions to the surface or the transport of chloride–copper complexes,43 and the depressed capacitive loop has been ascribed to roughness and in-homogeneities on the surface during corrosion.44 This behaviour is unchanged by the presence of the inhibitor, indicating the activation-controlled nature of the reaction throughout a one-charge transfer process. All EIS spectra were analyzed by the equivalent circuit in Fig. 3, which shows a single transfer reaction and fits well with our experimental results. Rs represents the ohmic resistance of the solution and Rct represents the charge transfer resistance whose value is an estimation of electron transfer across the surface and is contrary relative to corrosion rate. The constant phase element, CPE, is introduced in the circuit instead of a pure double layer capacitor to give a more accurate fit.45 The impedance of the CPE is expressed by the eqn (3):| ZCPE = [Y0(jw)n]−1 | (3) |
 | ||
| Fig. 2 The Nyquist plots of copper in hydrochloric acid 6.0 M with the various concentrations of: (a) NBPA, (b) NCMBPA. | ||
 | ||
| Fig. 3 Equivalent circuit model of impedance spectra in (a) blank, (b) the different concentration of inhibitors. | ||
The experiments are repeated three times and fitted spectra are listed in Table 1. The data of Table 1 show that the Rs values are very small contrast to the Rct values. Rct is a measure of electron transfer across the surface and is inversely proportional to corrosion rate. The Rct values increased with inhibitor concentrations may suggest the formation of a protective layer on the electrode surface. This layer makes a barrier for mass and charge-transfer.46 The Rct value of the blank solution is about 98.6 Ω cm2, while the Rct for NBPA inhibited solution increases to about 847.4 Ω cm2 and for NCMBPA inhibited solution increase about 1257.8 Ω cm2. The results show the inhibited molecules on the copper surface might be a barrier to the communication between oxide species and ionic solution.
| Inhibitor | Concentration (M) | Rs (Ω cm2) | Rct (Ω cm2) | Y0 (μF cm−2) | n | η% |
|---|---|---|---|---|---|---|
| Blank | — | 3.3 ± 0.041 | 98.6 ± 0.10 | |||
| NBPA | 5 × 10−4 | 2.2 ± 0.059 | 603.89 ± 0.59 | 0.001860 ± 0.6 × 10−6 | 0.49 ± 0.0053 | 84.0 ± 0.8 |
| 1 × 10−3 | 2.3 ± 0.013 | 687.77 ± 1.5 | 0.001060 ± 0.6 × 10−6 | 0.52 ± 0.0059 | 86.0 ± 1.2 | |
| 5 × 10−3 | 2.0 ± 0.019 | 734.19 ± 2.3 | 0.000974 ± 0.3 × 10−6 | 0.55 ± 0.0059 | 87.0 ± 0.9 | |
| 1 × 10−2 | 2.0 ± 0.039 | 847.44 ± 3.6 | 0.000647 ± 0.7 × 10−6 | 0.56 ± 0.0059 | 88.0 ± 1 | |
| NCMBPA | 5 × 10−4 | 1.3 ± 0.059 | 580.20 ± 1.5 | 0.00270 ± 0.6 × 10−6 | 0.48 ± 0.0053 | 83.0 ± 0.8 |
| 1 × 10−3 | 1.5 ± 0.059 | 681.00 ± 2.1 | 0.00112 ± 0.6 × 10−6 | 0.54 ± 0.0059 | 86.0 ± 1.1 | |
| 5 × 10−3 | 1.8 ± 0.053 | 866.90 ± 3.4 | 0.00110 ± 0.6 × 10−6 | 0.54 ± 0.0059 | 89.0 ± 0.9 | |
| 1 × 10−2 | 2.1 ± 0.053 | 1257.8 ± 9.6 | 0.00068 ± 0.7 × 10−6 | 0.55 ± 0.0059 | 92.0 ± 1 |
3.2. Potentiodynamic polarization measurements
The potentiodynamic polarization curves of copper in 6.0 M HCl with and without different concentrations of NBPA and NCMBPA are shown in Fig. 4. As shown in the figures, the presence of inhibitors causes a major decrease in the corrosion current. Decrease of current densities indicates in both anodic and cathodic Tafel curves. This phenomenon indicates that both anodic metal dissolution and cathodic hydrogen evolution reaction are suppressed, meanwhile, the inhibition of these reactions is more pronounced with the increasing inhibitor concentration.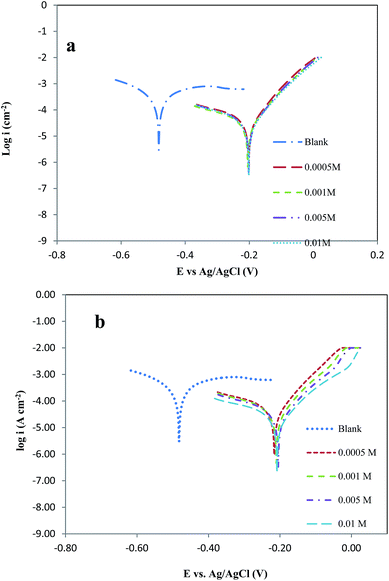 | ||
| Fig. 4 Polarization curves for copper in hydrochloric acid 6.0 M solution with the (a) NBPA, (b) NCMBPA, in different concentrations. | ||
The values of corrosion potential (Ecorr), cathodic Tafel slope (bc), anodic Tafel slope (ba), corrosion current (icorr) and the inhibition efficiency (η) are given in Table 2. These values are getting by extrapolating the Tafel lines to the related corrosion potentials.
| Inhibitor | Concentration (M) | ba (mV dec−1) | −bc (mV dec−1) | −E vs. Ag/AgCl (3 M Cl−) (mV) | icorr (μA cm−2) | η% |
|---|---|---|---|---|---|---|
| Blank | — | 131.2 ± 1 | 120.4 ± 1 | 482.1 ± 3 | 230.1 ± 0.07 | — |
| NBPA | 5 × 10−4 | 223.3 ± 1 | 106.5 ± 2 | 201.9 ± 2 | 47.3 ± 0.04 | 79.0 ± 0.6 |
| 1 × 10−3 | 225.6 ± 2 | 107.4 ± 1 | 198.4 ± 1 | 45.4 ± 0.05 | 80.0 ± 0.2 | |
| 5 × 10−3 | 195.1 ± 1 | 102.3 ± 4 | 197.7 ± 1 | 36.3 ± 0.01 | 84.0 ± 0.2 | |
| 1 × 10−2 | 171.0 ± 2 | 102.4 ± 1 | 201.7 ± 2 | 32.6 ± 0.06 | 86.0 ± 0.5 | |
| NCMBPA | 5 × 10−4 | 212.4 ± 1 | 124.9 ± 1 | 215.6 ± 1 | 62.4 ± 0.05 | 73.0 ± 0.1 |
| 1 × 10−3 | 133.5 ± 3 | 100.0 ± 3 | 205.6 ± 3 | 33.7 ± 0.02 | 85.0 ± 0.3 | |
| 5 × 10−3 | 123.1 ± 2 | 98.7 ± 1 | 204.2 ± 1 | 28.5 ± 0.02 | 88.0 ± 0.1 | |
| 1 × 10−2 | 83.4 ± 1 | 59.8 ± 1 | 185.2 ± 1 | 16.8 ± 0.01 | 93.0 ± 0.9 |
Examination of Table 2 shows that values of the corrosion current of copper in the inhibited solutions are lesser than that in the bare solution, and with the increasing concentration of Schiff's base inhibitors, the inhibition efficiency is increased. The inhibition efficiency finds from polarization experiments is created opposite the order: (NBPA) < (NCMBPA).
However, the difference of the inhibition efficiency between the two inhibitors is high, which can be featured to the difference between the structures of the two molecules. Alternatively, synergistic effect shows the increase in the efficiency of a corrosion inhibitor in the presence of a derivative species. The synergism effect has been reported due to the increased surface coverage on account of ion pair contacts between an organic cation and the halide anion. In inhibiting solution halide ion initially adsorbs on the corroding surface with making oriented dipoles and it eases the adsorption of inhibitor cations on the dipoles, consequently. Halide ions, especially Cl− can increase the speed or inhibit the copper corrosion. The synergism among the Cl− ions and organic inhibitors is very well shown. The purpose of protection is the protection in the presence of both the halide ion and the organic inhibitor in solution.
The inhibitor classified as cathodic or anodic type, if the displacement in Ecorr is exceeds 85 mV with respect to Ecorr of the blank and if the displacement in Ecorr is below 85 mV, the inhibitor can be mixed type.47 In the presence of NBPA and NCMBPA, the corrosion potential of copper shifted to the positive side more than 85 mV (vs. Ag/AgCl (3.0 M Cl−)). The NBPA and NCMBPA act can be understood as anodic type inhibitors.
3.3. Adsorption isotherm
The adsorption of inhibitor molecules is one of the most important topics in corrosion researches. Its importance comes from not only due to its ability to provide structural information of the double layer but also due to the thermodynamic information can provide. There are two categories from the adsorption of organic molecules on the electrode surface. First, adsorb the molecules with keeping their chemical individuality. This interaction is rather feeble. This is a reversible type of adsorption. On the contrary, the interaction among molecule and the surface may be very strong. This phenomenon may cause a charge transfer between them and results new species form. This is the irreversible type adsorption.Adsorption isotherms can offer the important information on the relations between the active sites on copper surface and the inhibitor molecules. The degrees of surface coverage (θ) for different concentrations of inhibitor in 6.0 M HCl solution have been calculated from the consequences of potentiodynamic polarization or EIS measurements with the eqn 4:48,49
 | (4) |
In this paper, with the intention of identify the nature and the strength of adsorption a series of experimental adsorption isotherms were obtained as shown in Fig. 5. It is clear that all linear correlation coefficients (r) are approximately equal to 1, and the slope values are also close to 1, which shows the adsorption of NBPA and NCMBPA on copper surface obeys Langmuir adsorption isotherm. It is represented by the eqn (5):
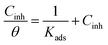 | (5) |
The Kads is the adsorption equilibrium constant, which is −0.85, −0.92 reveals more proficient adsorption thus better inhibition efficiency of NCMBPA on the copper surface.50 The equilibrium constant for the adsorption–desorption process is concerned to the standard free energy of adsorption ΔG ads as said by the eqn (6):
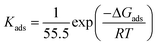 | (6) |
In general, if the values of ΔG ads are below the value of −20 kJ mol-1, they are consistent with the electrostatic interaction between the charged molecules and the charged metal (physical adsorption), while those above −40 kJ mol−1 are associated with chemisorption as a result of sharing or transfer of electrons from organic species to the metal surface to form a metal bond.51–53
As mentioned in above, the calculated value for ΔGads is −33.7 kJ mol−1 and −32.6 kJ mol−1 indicating that the adsorption mechanism of NBPA and NCMBPA on copper is the chemisorption and physisorption.54
3.4. Effect of temperature
The study on the temperature effect of inhibition efficiency from acid-metal reaction is very difficult, for the reason that several changes take place on the metal surface, for example quick etching desorption of inhibitor and the inhibitor may undergo decomposition.55The effect of the temperature on the corrosion rate was studied in 6.0 M HCl both in the absence and presence of 0.01 M of the inhibitor. Consequently, the polarization analysis was carried out at different temperatures from 25 °C to 65 °C in the absence and presence of 0.01 M inhibitors. The electrochemical factors were extracted and summarized in Table 4. The results in Table 3 show that the corrosion (η) increases with increasing temperature in the absence and presence of inhibitor but the η increases more quickly with temperature in the uninhibited solution. It is for reason that higher temperature increases the speed of hot-movement of the organic molecules and weakens the adsorption ability of inhibitor on the metal surface.56,57
| Inhibitor | Kads (×103) | R2 | ΔGads (kJ mol−1) | Ea (kJ mol−1) | ΔH*ads (kJ mol−1) | ΔS*ads (J mol−1 K−1) |
|---|---|---|---|---|---|---|
| Blank | — | — | — | 28.18 | 25.7 | −128.7 |
| NBPA | 20 | 0.99 | −33.7 | 32.18 | 26.3 | −119.2 |
| NCBMPA | 12.5 | 0.99 | −32.6 | 36.86 | 35.2 | −102.3 |
| Inhibitor | Temperature (°C) | ba (mV dec−1) | −bc (mV dec−1) | −E vs. Ag/AgCl (3 M Cl−) (mV) | icorr (μA cm−2) | η% |
|---|---|---|---|---|---|---|
| Blank | 35 | 123.1 ± 1 | 167.5 ± 2 | 451.0 ± 1 | 202.2 ± 0.07 | — |
| 45 | 109.6 ± 2 | 104.7 ± 1 | 482.8 ± 1 | 213.1 ± 0.07 | — | |
| 55 | 153.9 ± 2 | 198.5 ± 1 | 453.5 ± 1 | 260.6 ± 0.04 | — | |
| 65 | 130.9 ± 1 | 204.8 ± 3 | 458.5 ± 2 | 310.0 ± 03 | — | |
| NBPA | 35 | 159.8 ± 1 | 95.80 ± 2 | 184.8 ± 1 | 42.60 ± 0.04 | 79.0 ± 1.2 |
| 45 | 64.90 ± 3 | 318.0 ± 2 | 423.0 ± 2 | 64.20 ± 0.01 | 70.0 ± 1.4 | |
| 55 | 81.00 ± 2 | 98.30 ± 1 | 440.2 ± 3 | 90.90 ± 0.05 | 65.0 ± 0.8 | |
| 65 | 123.9 ± 1 | 249.4 ± 1 | 430.2 ± 1 | 120.8 ± 0.07 | 61.0 ± 1.6 | |
| NCBMPA | 35 | 97.90 ± 1 | 77.00 ± 1 | 201.4 ± 1 | 31.40 ± 0.03 | 84.0 ± 1.3 |
| 45 | 107.3 ± 2 | 138.1 ± 3 | 337.9 ± 2 | 56.40 ± 0.02 | 74.0 ± 1.1 | |
| 55 | 46.60 ± 3 | 71.90 ± 1 | 446.3 ± 1 | 80.70 ± 0.03 | 69.0 ± 0.5 |
The energy of activation for the corrosion process was calculated from the Arrhenius equation (eq (7)):
 | (7) |
The high values of Ea show that more energy barrier for the corrosion reaction in the presence of the inhibitor is achieved. Therefore the adsorbed inhibitor molecules avoided charging or mass transferring from the copper surface.
The Thermodynamic parameters like enthalpy (ΔH*) of corrosion process and entropy (ΔS*) were calculated with the transition state eqn (8):
 | (8) |
 | ||
| Fig. 6 The plots of ln(icorr/T) vs. 1000/T for copper in hydrochloric acid 6.0 M in the absence and presence of 0.01 M of inhibitors. | ||
 | ||
| Fig. 7 The plots of ln(icorr) vs. 1000/T for copper in hydrochloric acid 6.0 M in the absence and presence of 0.01 M of inhibitors. | ||
3.5. Quantum chemical calculations
Quantum chemical calculations were performed in order to analyze the effect of molecular structure and also electronic parameters on present inhibitor performance. The computed quantum chemical parameters including the energy of highest occupied molecular orbital (EHOMO (eV)), energy of lowest unoccupied molecular orbital (ELUMO (eV)), HOMO–LUMO energy gap (ΔE (eV)) and dipole moment (μ (D)) have been presented in Table 5. According to the frontier molecular orbital theory, the formation of a transition state is due to an interaction between the frontier orbitals (HOMO and LUMO) of reactants.63 The HOMO is known as an orbital which could act as an electron donor because it is the outermost orbital containing electrons, having highest energy level. On the contrary, The LUMO is the innermost orbital which can accept electrons through its vacant room.64 Basically, EHOMO and ELUMO are directly related to the ionization potential and the electron affinity of an organic molecule, respectively. The HOMO–LUMO gap, ΔE (eV), is an important stability index so that a large ΔE (eV) value implies high stability for the molecule in chemical reactions.65 Inspection of Table 5 shows nearly similar value for EHOMO for both compounds while NCMBPA has lower ELUMO (ev) than NBPA. It may be deduced that both inhibitors have an equal ability to offer electrons to the unoccupied d-orbital of the copper, however the higher ability of accepting the electron is expected for NCMBPA in comparison with NBPA. Considering the ΔE (eV) values, the higher tendency in order to adsorb onto metal surface is measured for NCMBPA, having lower ΔE (eV) compared to NBPA. In the case of the dipole moment, it seems to be no agreement in the literature concerning the correlation between the dipole moment and the inhibition effectiveness, as yet. Since water is a polar solvent, there is a higher tendency for molecules with similar size to adsorb onto the metal surface for those who have a lower dipole moment.66 In this study, NBPA has a lower dipole moment and molecular size in comparison with NCMBPA while both inhibitors show good inhibition efficiency. According to the above discussion, it may be concluded that both inhibitors, considering their molecular weight and dipole moment, have a tendency to adsorb onto metal surface. For instance, the higher molecular weight and dipole moment make an affinity for NCMBPA to leave the solution and get adsorbed onto surface. It should be noted that molecular electronic parameters are not confident parameters which certainly determines the corrosion inhibition performance because corrosion inhibition is a complex phenomenon.| Method | Parameters | Inhibitor | |
|---|---|---|---|
| NBPA | NCMBPA | ||
| B3LYP/6-311G** | EHomo (eV) | −0.2362 | −0.2343 |
| ELumo (eV) | −0.0771 | −0.0819 | |
| ΔE (eV) | 0.1591 | 0.1524 | |
| μ (D) | 3.4076 | 4.5691′ | |
| PCM | EHomo (eV) | −0.2362 | −0.2343 |
| ELumo (eV) | −0.0771 | −0.0819 | |
| ΔE (eV) | 0.1591 | 0.1524 | |
| μ (D) | 3.4076 | 4.5691 | |
| CPCM | EHomo (eV) | −0.2398 | −0.2376 |
| ELumo (eV) | −0.0811 | −0.0848 | |
| ΔE (eV) | 0.1587 | 0.1528 | |
| μ (D) | 4.0109 | 4.6504 | |
In order to validate such correlations between calculated quantum parameters (eqn (9)) of experimental inhibition efficiencies for the two Schiff bases, the non-linear multiple regressions were obtained by B3LYP/6-311G method. The results of this calculation are shown in Table 6.
 | (9) |
The calculated efficiencies (Ecal%) from the eqn (9) at different concentrations of these compounds show good correlations to experimental efficiencies (Eexp%) with correlation coefficients R2 = 0.89 for NBPA and R2 = 0.91 for NCMBPA.67 The plot of the experimental and the calculated inhibition efficiency (from COSMO method) for NBPA and NCMBPA compounds were presented in Fig. 8. The similar plots were obtained for the other methods (not shown).
 | ||
| Fig. 8 The correlation between experimental inhibition efficiency and calculated inhibition efficiency obtained from COSMO method. | ||
3.6. SEM analysis
The SEM images of copper specimens exposed to 6.0 M HCl for 3 h with and without 0.01 M NBPA and NCMBPA are given in Fig. 9. It can be obviously observed from Fig. 8b that the surface morphology was severely corroded in the absence of inhibitors. However, in the presence of inhibitors, the destruction of copper surfaces is clearly reduced and even the polishing lines can be observed (Fig. 9c and d). This observation clearly proves that NBPA and NCMBPA can show good inhibiting ability on copper surface. The inhibitors may form protective films adsorbed on metal surface, resulting in a decrease in the contact between copper and the corrosive solution. | ||
| Fig. 9 The SEM images of the copper surface after 3 h immersion period (a) before corrosion, (b) 6.0 M HCl, (c) 6.0 M HCl + 0.01 M NBPA, (d) 6.0 M HCl + 0.01 M NCMBPA. | ||
4. Conclusion
The studied two N-benzylidene derivative compounds are professional inhibitors for the corrosion of copper in 6.0 M hydrochloric acid solutions. Polarization curves show that corrosion potentials shift toward the anodic region at presence of the examined two N-benzylidene derivative compounds.The results of impedance demonstrate that as the additive concentration is increased the polarization resistance increases. Adsorption of the inhibitor molecules onto the copper surface follow by the Langmuir isotherm. The activation parameters of corrosion process such as activation energy (Ea), activation enthalpy (ΔH*) and activation entropy (ΔS*), are calculated from the dependence of corrosion rates on the temperature.
Through the quantum calculation methods used, the B3LYP/6-311G** and COSMO methods shows that the best correlation for some of the parameters studied and experimental results. The fact that inhibition efficiencies increase when the ELUMO decrease shows that feedback bonds form between the Schiff bases and copper surface. Quantum chemical calculations were further applied to explain the experimental results.45,68
Acknowledgements
We are grateful to University of Kashan for supporting this work with Grant 256450-12.References
- G. Tansug, T. Tuken, E. S. Giray, G. Fındıkkıran, G. Sıg ırcık, O. Demirkol and M. Erbil, Corros. Sci., 2014, 84, 21–29 CrossRef CAS PubMed.
- M. M. Antonijevic and B. M. B. Petrovic, Int. J. Electrochem. Sci., 2008, 3, 1–28 CAS.
- M. M. Antonijevic, S. M. Milic and M. B. Petrovic, Corros. Sci., 2009, 51, 1228–1237 CrossRef CAS PubMed.
- S. Sudheer and M. A. Quraishi, Corros. Sci., 2013, 70, 161–169 CrossRef PubMed.
- M. Scendo, Corros. Sci., 2005, 47, 1738–1749 CrossRef CAS PubMed.
- E. S. Zocs, G. Vastag, A. Shaban and E. Kalman, Corros. Sci., 2005, 47, 893–908 CrossRef PubMed.
- E. M. Sherif and S.-M. Park, Electrochim. Acta, 2006, 51, 4665–4671 CrossRef CAS PubMed.
- M. Behpour, S. M. Ghoreishi, M. Khayat Kashani and N. Soltani, Corros. Sci., 2011, 53, 2489–2501 CrossRef CAS PubMed.
- M. Behpour, S. M. Ghoreishi, N. Soltani and M. Salavati-Niasari, Corros. Sci., 2009, 51, 1073–1089 CrossRef CAS PubMed.
- S. Li, H. Ma, S. Lie, R. Yu, S. Chen and D. Liu, Corros. Sci., 1998, 54, 947–953 CrossRef CAS.
- T. T. Qin, J. Li, H. Q. Luo, M. Li and N. B. Li, Corros. Sci., 2011, 53, 1072–1078 CrossRef CAS PubMed.
- M. Behpour, S. M. Ghoreishi, N. Soltani and M. Salavati-Niasari, Corros. Sci., 2010, 52, 4046–4057 CrossRef CAS PubMed.
- M. G. Fontana and N. D. Greece, Corrosion Engineering, McGraw-Hill, New York, 1986 Search PubMed.
- Y. Abboud, A. Abourriche, T. Saffaj, M. Berrada, M. Charrouf, A. Bennamara, N. Al Himidi and H. Hannache, Mater. Chem. Phys., 2007, 105, 1–142 CrossRef CAS PubMed.
- M. Lebrini, M. Lagreńee, H. Vezin, L. Gengembre and F. Bentis, Corros. Sci., 2005, 47, 485 CrossRef CAS PubMed.
- I. Dehri and M. Ozcan, Mater. Chem. Phys., 2006, 98, 316 CrossRef CAS PubMed.
- F. Zucchi, G. Trabanelli and M. Fonsati, Corros. Sci., 1996, 38, 2019–2029 CrossRef CAS.
- M. Finšgar, Corros. Sci., 2013, 77, 350–359 CrossRef PubMed.
- H. O. Curkovic, E. Stupnisek-Lisac and H. Takenouti, Corros. Sci., 2009, 51, 2342–2348 CrossRef PubMed.
- R. Vera, F. Bastidas, M. Villarroel, A. Oliva, A. Molinari, D. Ramirez and R. del Rio, Corros. Sci., 2008, 50, 729–736 CrossRef CAS PubMed.
- F. Zucchi, G. Trabanelli and C. Monticelli, Corros. Sci., 1996, 38, 147–154 CrossRef CAS.
- K. F. Khaled, Mater. Chem. Phys., 2011, 125, 427–433 CrossRef CAS PubMed.
- D. Gopi, K. M. Govindaraju, V. C. A. Prakash, D. M. A. Sakila and L. Kavitha, Corros. Sci., 2009, 51, 2259–2265 CrossRef CAS PubMed.
- G. Kear, B. D. Barker and F. C. Walsh, Corros. Sci., 2004, 46, 109–135 CrossRef CAS.
- M. Scendo, Corros. Sci., 2008, 50, 1584–1592 CrossRef CAS PubMed.
- F. Rosalbino, R. Carlini, F. Soggia, G. Zanicchi and G. Scavino, Corros. Sci., 2012, 58, 139–144 CrossRef CAS PubMed.
- M. B. Valcarce and M. Vazquez, Corros. Sci., 2010, 52, 1413–1420 CrossRef CAS PubMed.
- A. A. Farag and M. A. Hegazy, Corros. Sci., 2013, 74, 168–177 CrossRef CAS PubMed.
- M. Gopiraman, N. Selvakumaran, D. Kesavan and R. Karvembu, Prog. Org. Coat., 2012, 73, 104–111 CrossRef CAS PubMed.
- S. T. Zhang, Z. H. Tao, W. H. Li and B. R. Hou, Appl. Surf. Sci., 2009, 255, 6757–6763 CrossRef CAS PubMed.
- M. Lashgari, M. Arshadi and S. Miandari, Electrochim. Acta, 2010, 55, 6058–6063 CrossRef CAS PubMed.
- A. Yuce and G. Kardas, Corros. Sci., 2012, 58, 86–94 CrossRef PubMed.
- S. Issaadi, T. Douadi, A. Zouaoui, S. Chafaa, M. A. Khan and G. Bouet, Corros. Sci., 2011, 53, 1484–1488 CrossRef CAS PubMed.
- M. A. Migahed, A. A. Farag, S. M. Elsaed, R. Kamal and H. Abd El-bary, Chem. Eng. Commun., 2012, 199, 1335–1356 CrossRef CAS.
- I. Ahamad, R. Prasad and M. A. Quraishi, Mater. Chem. Phys., 2010, 124, 1155–1165 CrossRef CAS PubMed.
- H. A. Mohamed, A. A. Farag and B. M. Badran, J. Appl. Polym. Sci., 2010, 117, 1270–1278 CAS.
- S. Safak, B. Duran, A. Yurt and G. Turkoglu, Corros. Sci., 2012, 54, 251–259 CrossRef CAS PubMed.
- A. A. Farag and M. R. Noor El-Din, Corros. Sci., 2012, 64, 174–183 CrossRef CAS PubMed.
- K. C. Emregul and O. Atakol, Mater. Chem. Phys., 2004, 83, 373–379 CrossRef CAS PubMed.
- I. B. Obot, N. O. Obi-Egbedi and S. A. Umoren, Corros. Sci., 2009, 51, 276–282 CrossRef CAS PubMed.
- H. Naeimi and Z. S. Nazifi, Bull. Chem. Soc. Ethiop., 2013, 27, 143–149 CAS.
- A. Klamt and G. Schuurmann, J. Chem. Soc., Perkin Trans. 2, 1993, 799–805 RSC.
- W. Deng, P. Lin, Q. Li and G. Mob, Corros. Sci., 2013, 74, 44–49 CrossRef CAS PubMed.
- S. S. de Assunção, A. Pereira, M. M. Pegas, T. L. Fernández, M. Magalhães, T. G. Schöntag, D. C. Lago, L. F. de Senna and E. D'Elia, Corros. Sci., 2012, 65, 360–366 CrossRef PubMed.
- M. Behpour, S. M. Ghroreishi, N. Soltani, M. Salavati-Niasari, M. Hamadanian and A. Gandomi, Corros. Sci., 2008, 50, 2172–2181 CrossRef CAS PubMed.
- K. F. Khaled, Mater. Chem. Phys., 2008, 112, 104–111 CrossRef CAS PubMed.
- M. Behpour, N. Mohammadi and E. Alian, J. Iron Steel Res. Int., 2014, 21, 121–124 CrossRef.
- L. Li, Q. Qu, W. Bai, F. C. Yang, Y. J. Chen, S. W. Zhang and Z. T. Ding, Corros. Sci., 2012, 59, 249–257 CrossRef CAS PubMed.
- A. Y. Musa, R. T. T. Jalgham and A. B. Mohamad, Corros. Sci., 2012, 56, 176–183 CrossRef CAS PubMed.
- A. Popova and M. Christov, J. Univ. Chem. Technol. Metall., 2008, 43, 37–47 CAS.
- A. Yurt, G. Bereket, A. Kivrak, A. Balaban and B. Erk, J. Appl. Electrochem., 2005, 35, 1025–1032 CrossRef CAS.
- A. M. Fekry and R. R. Mohamed, Electrochim. Acta, 2010, 55, 1933–1939 CrossRef CAS PubMed.
- V. R. Saliyan and A. V. Adhikari, Corros. Sci., 2008, 50, 55–61 CrossRef CAS PubMed.
- L. M. Vracar and D. M. Drazic, Corros. Sci., 2002, 44, 1669–1680 CrossRef CAS.
- J. Alijourani, K. Raeissi and M. A. Golozar, Corros. Sci., 2009, 51, 1836–1843 CrossRef PubMed.
- D. Daoud, T. Douadi, S. Issaadi and S. Chafaa, Corros. Sci., 2014, 79, 50–58 CrossRef CAS PubMed.
- W. Li, X. Zhao, F. Liu and B. Hou, Corros. Sci., 2008, 50, 3261–3266 CrossRef CAS PubMed.
- L. Larabi, O. Benali and Y. Harek, Mater. Lett., 2007, 61, 3287–3293 CrossRef CAS PubMed.
- A. Popova, E. Sokolova, S. Raicheva and M. Christov, Corros. Sci., 2003, 45, 331–345 CrossRef.
- T. Szauer and A. Brandt, Electrochim. Acta, 1981, 26, 1209 CrossRef CAS.
- M. Bouklah, N. Benchat, B. Hammouti, A. Aouniti and S. Kertit, Mater. Lett., 2006, 60, 2840–2843 CrossRef PubMed.
- E. A. Noor and A. H. Al-Moubaraki, Mater. Chem. Phys., 2008, 110, 145–154 CrossRef CAS PubMed.
- M. Finsgar, A. Lesar, A. Kokalj and I. Milosev, Electrochim. Acta, 2008, 53, 8287–8297 CrossRef CAS PubMed.
- H. Ju, Z. Kai and Y. Li, Corros. Sci., 2008, 50, 865–871 CrossRef CAS PubMed.
- G. Gece, Corros. Sci., 2008, 50, 2981–2992 CrossRef CAS PubMed.
- A. Kokalj, Electrochim. Acta, 2010, 56, 745–755 CrossRef CAS PubMed.
- A. Kokalj, S. Peljhan, M. Fins gar and I. Milosev, J. Am. Chem. Soc., 2010, 132, 16657–16668 CrossRef CAS PubMed.
- H. Ashassi-Sorkhabi, B. Shaabani and D. Seifzadeh, Appl. Surf. Sci., 2005, 239, 154 CrossRef CAS PubMed.
- J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999–3094 CrossRef CAS PubMed.
- M. Nadia Rega, G. Scalmani and V. Barone, J. Comput. Chem., 2003, 24, 669–681 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 851.16
851.16