Facile synthesis of Ag nanowire–rGO composites and their promising field emission performance†
Aneeya K. Samantaraa,
Dillip Kumar Mishraa,
Sachin R. Suryawanshib,
Mahendra A. More*b,
Ranjit Thapac,
Dattatray J. Late*d,
Bikash Kumar Jena*a and
Chandra Sekhar Rout*e
aCSIR-Institute of Minerals and Materials Technology, Bhubaneswar 751013, Odisha, India. E-mail: bikash@immt.res.in
bDepartment of Physics, Savitribai Phule Pune University Pune-411007, Maharashtra, India. E-mail: mam@physics.unipune.ac.in
cSRM Research Institute, SRM University, Kattankulathur, Chennai 603 203, Tamil Nadu, India
dPhysical & Materials Chemistry Division, CSIR-National Chemical Laboratory, Dr. Homi Bhabha Road, Pune 411008, Maharashtra, India. E-mail: datta099@gmail.com; dj.late@ncl.res.in
eSchool of Basic Sciences, Indian Institute of Technology, Bhubaneswar 751013, Odisha, India. E-mail: csrout@iitbbs.ac.in
First published on 7th April 2015
Abstract
Crystalline, ultra long silver nanowires (Ag NWs), few-layered rGO (reduced graphene oxide) and their rGO–Ag NW nanocomposite have been synthesized using a polyol reflux technique under optimized experimental conditions. The field emission performance of the rGO–Ag NW nanocomposite, rGO and Ag NW emitters was investigated. The turn on field required to draw an emission current density of ∼1 μA cm−2 was found to be ∼5.00, 3.92 and 2.40 V μm−1 for the Ag NW, rGO and rGO–Ag NW nanocomposite emitters, respectively. The combined contribution of the sharp edges of the thin graphene sheets and high aspect ratio of the Ag nanowires, and their synergetic effect in the rGO–Ag NW nanocomposite, are responsible for the enhanced field emission behavior. First-principles density functional calculations show that the enhanced field emission may also be due to the overlapping of the electronic structures of the Ag NWs and rGO nanosheets.
1. Introduction
The shape and size of nanomaterials play a substantial role in their application in different fields i.e. from electronics to biology. In order to enhance their behavior, a surface architecture is required in which the shape modulation becomes the dominating factor over the tunable size. For which, so many efforts have been devoted towards the development of different shaped nanomaterials with controlled morphology.1–6 Shape and size controlled nanomaterials of different structures, such as one-dimensional (1D) nanorods/nanowires, two-dimensional (2D) nanoplates, three-dimensional (3D) nanocubes and so forth, have been explored.3,4,7–9 Among these structures, 1D nanowires have been attractive for vast applications in electronics, photovoltaics, biological imaging etc.10,11 Compared to other metallic nanostructures (Zn, Cu, W, Sn etc.), Ag nanowires (NWs) have been generally applied for the development of sensors, catalysis, memory storage devices and so forth, due to their dominant electrical conductivity, thermal stability and structure-dependent optical properties.12–16 Recently, networks of Ag NWs and graphene hybrid films were observed to present special features and usefulness in optical and electronic devices, catalysis, sensors, etc.17–19 These hybrid films showed potential to replace Indium Tin Oxide (ITO) electrodes, due to a high optical transparency of 94%, low sheet resistance of 33 ohm sq−1 and excellent stability upon exposure to the atmosphere, mechanical pressure and bending.19Some methods have been documented for the synthesis of Ag NWs using template based processes.7,8,20,21 However, these methods are limited by the yield obtained for their application. Therefore, solution phase reactions for high yield synthesis of Ag NWs have followed. Here, we synthesize the Ag NWs by following a solution phase route, using AgNO3 as the metal precursor that is reduced to form the Ag NWs in an ethylene glycol medium in the presence of polyvinyl pyrrolidone (PVP). This method, called the polyol process, has been broadly adopted for the synthesis of different metal nanoparticles.7,8 On the other hand, the support materials play a vital role in providing stability to the nanoparticles and trigger their efficiency for many applications towards catalysis, electro-catalysis, the development of different electronic devices and so forth.22–27 Among the various materials employed as supports, carbon based materials like graphene, carbon nanotubes (single/multi-walled), fullerene, etc. are broadly used. Graphene is a single atom-thick 2D carbon material comprising a honeycomb structure of sp2 bonded carbon atoms. It has very high electrical conductivity, thermal conductivity, chemical inertness and a high surface area to hold the bare nanostructures. Further, the increase in performance of a graphene–Ag composite authenticates their development and application.21 Therefore, a facile, single step synthetic approach for the development of such types of composites is still desired.
In this report, we have used a facile single step polyol method for the high yield synthesis of reduced graphene oxide–Ag NW (rGO–Ag NW) hybrids. The high electrical conductivity of the Ag/graphene hybrids attracted us to investigate its field emission performance for possible applications in vacuum micro-/nanoelectronic devices. We have investigated the field emission properties of the rGO–Ag NWs and compared their performance with the bare Ag NW and reduced graphene (rGO) emitters.
2. Experimental section
2.1 Materials and synthesis
The graphite powder, AgCl, AgNO3, KBr, and ethylene glycol were purchased from Sigma-Aldrich. KMnO4 and polyvinyl pyrrolidone (PVP) were collected from Himedia chemicals. All other chemicals were of analytical grade and used without any further purification. In all the experiments, Millipore water (18 Ωm) was used for preparation of the solution.2.2 Synthesis of graphene oxide
The graphene oxide (GO) synthesis was carried out by following the modified Hummer’s method.28 In a typical procedure, 1 g of graphite powder and 25 ml of concentrated H2SO4 were taken in a conical flask and stirred for 10 minutes in an ice bath. Then, 3.5 g of KMnO4 was added slowly and the whole mixture was allowed to stir for two hours in a water bath at room temperature. On completion of the two hours, the conical flask containing the aforesaid mixture was again placed in the ice bath, followed by the addition of 50 ml of deionised water. Then a sufficient amount of 30% H2O2 was added, until the effervescence of gas ceased. The obtained brown colored suspension of GO was filtered and repeatedly washed with 0.1 M HCl and water for the complete removal of SO42− ions. The sample was freeze-dried and stored for future use.2.3 Synthesis of reduced graphene oxide (rGO)
20 ml of ethylene glycol was taken in a three-necked round bottom flask and heated to 170 °C. Once the temperature was maintained, 10 ml of an aqueous graphene oxide suspension (1 mg ml−1) was added drop-wise and the mixture was left to stir for the next 30 minutes. After that, a black colored suspension was collected by centrifugation and washed properly with ethanol.2.4 Synthesis of the Ag NWs
The Ag NWs were synthesized following a single step polyol method.29 In a typical procedure, a mixture of 0.668 g of polyvinyl pyrrolidone (PVP), 0.01 g of KBr and 20 ml of EG (ethylene glycol) was placed in a three-necked round bottom flask and heated to maintain a constant temperature of 170 °C. Then, 0.050 g of finely powdered AgCl was added slowly and the reaction was left for 3 to 4 minutes. 0.220 g of finely powdered AgNO3 was added slowly to the suspension. The heating was continued for the next 30 minutes. After that, the flask containing the solution was immediately placed in an ice bath to cool the solution and then stored for future use.2.5 Synthesis of rGO–Ag NWs
The synthesis of the rGO–Ag NW composite was carried out using the same synthetic procedure followed for the Ag NWs. 10 ml of a graphene oxide aqueous solution (1 mg ml−1) was added drop-wise, before placing the round bottom flask in the ice bath, and the solution was boiled for another 20 minutes.2.6 Characterization
The morphology of the as-prepared samples was studied by field emission scanning electron microscopy (FE-SEM) measurements at a 20 kV acceleration voltage, using a Neon 40 cross-beam system (M/S Carl Zeiss GmbH). The samples for SEM analysis were prepared on an aluminum foil and vacuum dried prior to the analysis. The transmission electron microscopy (TEM) and high resolution TEM analysis was performed on a 200 keV, JEOL JEM-2010 instrument. The X-ray diffraction patterns were recorded using a Bruker D8 Advance Diffractometer well equipped with a Ni filtered Cu Kα radiation of 0.154 nm wavelength (at 40 kV, 40 mA). The Raman studies of the samples were carried out using a Renishaw inVia Raman microscope with laser radiation excitation of 514 nm and a 50× objective at a 10 second exposure time.2.7 Field emission
The field emission (FE) current density (J) versus applied electric field (E) and the emission current (I) versus time (t) characteristics were measured in a planar ‘diode’ configuration at a base pressure of ∼1.0 × 10−8 mbar. A typical ‘diode’ configuration consists of a phosphor coated semitransparent screen (a circular disc having a diameter of ∼4 cm) as an anode. In order to investigate the FE properties, the rGO–Ag NW composite powder was sprinkled on a piece of carbon tape (radius ∼ 2.5 mm). The rGO–Ag NW composite sprinkled carbon tape was pasted on a stainless steel holder (diameter ∼4.5 mm), which acted as a cathode. The FE measurements were carried out at a fixed cathode–anode separation of ∼1 mm. The emission current was measured by a Keithely electrometer (6514) using a sweeping DC voltage applied to the cathode with a step of 40 V (0–40 kV, Spellman, U.S.). The FE current stability was recorded at different preset current values using a computer controlled data acquisition system. Special care was taken to avoid any current leakage, by using shielded cables and ensuring proper grounding. Before recording the FE measurements, pre-conditioning of the cathode was carried out by keeping it at ∼500 volts for 30 min, so as to remove loosely bound particles and/or contaminants through residual gas ion bombardment.2.8 Computational details
The first principles spin polarized calculations were performed using a Vienna Ab initio Simulation Package (VASP) that is based on density functional theory (DFT) calculations using the plane-wave basis set.30,31 Core electrons were described with the Projector Augmented Wave (PAW) method.32 The gradient-corrected functional, developed by Perdew, Burke, and Ernzerhof (PBE), was used to describe the electron exchange–correlation potentials.33 The kinetic energy cut-off was set to 400 eV. The k-point meshes were sampled using the scheme of Monkhorst–Pack. All of the structures were deemed to have fully relaxed when the total energy converged within 10−5 eV per atom and the maximum force converged within 0.001 eV Å−1.3. Results and discussion
3.1 FESEM, XRD, and Raman investigations
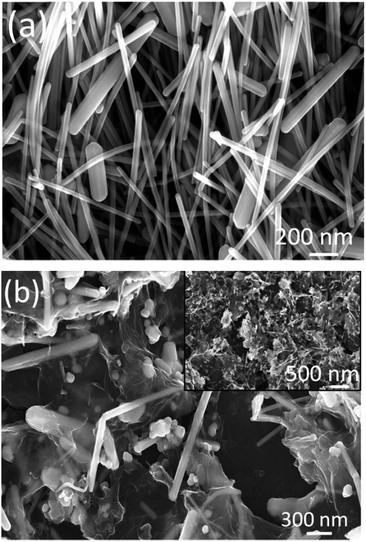
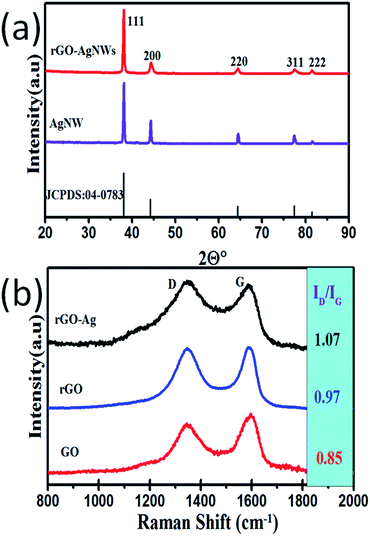
Here, we report the synthesis of Ag NWs by a polyol method. In this method, ethylene glycol acts both as a solvent and reducing agent. During the reflux reaction, the silver nitrate gets reduced, to form small sized silver nanoparticles, followed by the growth of larger particles through an Ostwald ripening process. With the assistance of PVP (which controls the growth rate of different faces of the silver by means of surface coordination), these larger particles were directed to grow in a definite direction to form nanowires, like the structures found in Fig. 1(a).8 The FESEM image (Fig. 1(a)) shows the formation of randomly distributed ultra long nanowires (length up to a few micrometer) having diameters in the range of 55 to 70 nm. It is clearly visible that the Ag NWs have a very smooth surface, and that some of them are protruding outside of the substrate surface. Fig. 1(b) shows the FESEM image of the rGO–Ag NW composite. As it can be seen, the Ag NWs are embedded in the rGO network. A typical XRD pattern of the rGO–Ag NW nanocomposite (Fig. 2(a)) exhibits a set of well-defined diffraction peaks, which imply its crystalline nature. The observed diffraction peaks could be indexed to the Ag NW (JCPDS card, no. # 04-0783) and rGO phases. Interestingly, the XRD pattern does not show diffraction peak(s) corresponding to other impurity phases, indicating a high purity of the as-synthesized product. Thus, the XRD analysis clearly reveals the formation of a high purity crystalline rGO–Ag NW nanocomposite phase under the prevailing experimental conditions.The as-synthesized GO and rGO were characterized using different techniques. The folds and wrinkles present in the GO sheets are clearly visible from the FESEM images (Fig. S1a and b, ESI†). The TEM images show the transparent nature of the GO sheets (Fig. S1c, ESI†). The existence of well distinguished diffraction points in the SAED pattern (Fig. S1d, ESI†) indicates the crystalline nature of the GO. This is also supported by the appearance of a sharp XRD peak (2θ = 10.43°) with an interlayer spacing of 0.76 nm (002 plane) (Fig. S2, ESI†). But in the case of rGO, this prominent XRD peak pattern disappeared and a small hump appeared at 26° (2θ). This observation revealed the efficient reduction of GO to form rGO.
Furthermore, Raman analysis of the samples was carried out to verify the efficient reduction of GO and the formation of the rGO–Ag NW composite (Fig. 2(b)). In the Raman spectra of GO and rGO, two prominent peaks appear clearly at 1346 cm−1 (D-band) and 1585 cm−1 (G-band), assigned to the in-plane vibration of the k-point phonons of A1g symmetry and first order scattering of the E2g phonon for the in phase stretching vibration of the sp2 carbon atoms, respectively.25,34 The intensity ratio of the D and G bands (ID/IG) was found to be increased from GO (0.85) to rGO (1.07), representing the formation of small sized sp2 domains in the rGO. This observation further confirms the formation of a rGO–Ag NW composite.
3.2 Field emission
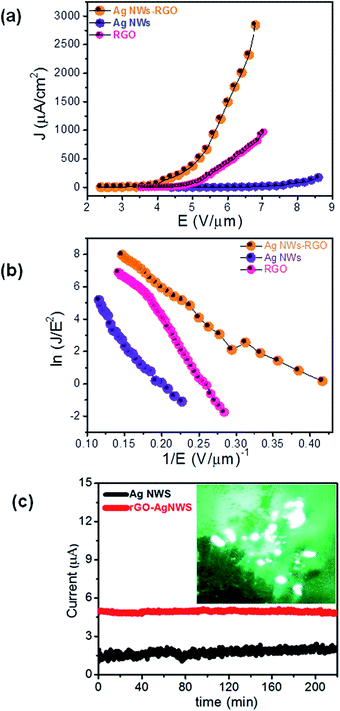
For comparison, herein we report the FE behaviour of the pristine rGO, Ag NW and rGO–Ag NW nanocomposite emitters. The characteristic field emission current density versus applied field (J–E) plots of the rGO, Ag NW and rGO–Ag NW nanocomposite emitters are depicted in Fig. 3(a). The values of the turn-on and threshold fields, defined as the field required to draw an emission current density of ∼1 μA cm−2 and ∼10 μA cm−2, respectively, are compiled in Table 1. Furthermore, a very high emission current density of ∼2.9 mA cm−2 was drawn from the rGO–Ag NW nanocomposite emitter at a relatively low applied field of ∼6.80 V μm−1 (for comparison, the field emission parameters of rGO, Ag NWs, the rGO–Ag NW and various previously reported metal/metal oxide–rGO nanocomposites are compiled in Table 1).| Composite | Turn-on field | Field enhancement factor (β) | Ref. |
|---|---|---|---|
| SnS2/rGO | 1 μA cm−2 at 2.65 V μm−1 | 3700 | 37 |
| WS2/rGO | 1 μA cm−2 at 2.0 V μm−1 | 2978 | 38 |
| Ag NWs | 1 μA cm−2 at 3.40 V μm−1 | 427 | 39 |
| Si–ZnO | 10 μA cm−2 at 7.8 V μm−1 | — | 40 |
| rGO | ∼3.92 V μm−1 | 1013 | Present study |
| Ag NWs | ∼5.0 V μm−1 | 1047 | Present study |
| rGO–Ag NWs | ∼2.40 V μm−1 | ∼1985 | Present study |
The observed lower values of the turn-on and threshold field are attributed to the high aspect ratio of the Ag NWs and sharp edges of the rGO sheets in the rGO–Ag NW nanocomposite, owing to their nanometric dimensions. Furthermore, as seen from the FESEM images of the hybrid nanocomposite (characterized by the presence of a few bare Ag NWs, and rGO nanosheets covering the Ag NWs), its FE behavior is due to the combined effect of the high aspect ratio of Ag NWs and the rGO nanosheets, along with synergic effects due to the formation of a heterostructure, resulting in modulation of the electronic properties. Furthermore it can be said that the hybrid nanocomposite emitter exhibits less field screening effects, and thus the high field enhancement at the ‘protruding’ Ag NWs and the atomically sharp edges of the rGO nanosheets is responsible for the lowering of the turn-on and threshold values.
The extraction of a very high emission current density (∼2.9 mA cm−2) at a relatively lower applied field (∼6.80 V μm−1) can be attributed to the modulation of the electronic properties of the hybrid nanocomposite. For field emission, such a unique surface topography of the emitter and enhanced electrical properties of the hybrid composite should have enhanced the emission behavior. As per the F–N theory, emission of electrons via tunneling through a deformation of the potential barrier is exponentially dependent on the work function (ϕ) of the emitter. The difference between the potential energy of an electron between the Fermi level (Ef) and vacuum level (Ev) is defined as (ϕ). The vacuum level is the potential energy that approaches a nearly constant value in the energy distributions at the vacuum region. Here, the 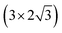 supercell of the Ag (111) surface (three layers) on the
supercell of the Ag (111) surface (three layers) on the 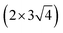 supercell of a graphene layer with minimal lattice mismatch has been considered, to estimate the work function of the hetero-structure (Ag (111)/graphene). A side view of the model structure is shown in Fig. 4(a). The electrostatic potential as a function of the coordinate along the c-axis of the Ag (111)/graphene surface is depicted in Fig. 4(b). The work function of the Ag (111)/graphene surface has been estimated to be about 4.25 eV, which is about 0.28 eV less than a pure graphene surface (ϕ = 4.53 eV). The estimated electrostatic potential is shown in Fig. 5.
supercell of a graphene layer with minimal lattice mismatch has been considered, to estimate the work function of the hetero-structure (Ag (111)/graphene). A side view of the model structure is shown in Fig. 4(a). The electrostatic potential as a function of the coordinate along the c-axis of the Ag (111)/graphene surface is depicted in Fig. 4(b). The work function of the Ag (111)/graphene surface has been estimated to be about 4.25 eV, which is about 0.28 eV less than a pure graphene surface (ϕ = 4.53 eV). The estimated electrostatic potential is shown in Fig. 5.
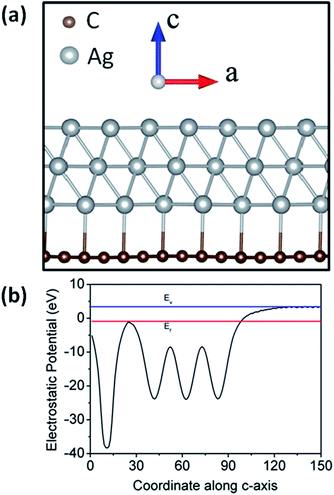 | ||
| Fig. 4 (a) Side view of Ag (111) on a graphene surface, (b) electrostatic potential energy average along the a and b axes, and plotted along the c-axis. Ev and Ef denote vacuum and Fermi energy. | ||
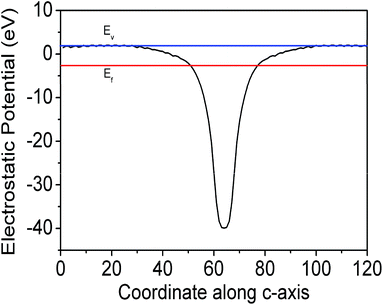 | ||
| Fig. 5 Electrostatic potential energy average of graphene, along the a and b axes and plotted along the c-axis. Ev and Ef denote vacuum and Fermi energy. | ||
The modified form of the Fowler–Nordehim (F–N) equation for multi-tip emitters deposited on flat substrates is as follows,31–36
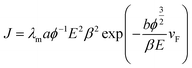 | (1) |
The Fowler–Nordheim (F–N) plots, derived from the observed J–E characteristics, are shown in Fig. 3(b) for the rGO, Ag NWs and rGO–Ag NW nanocomposite emitter. The ratio of the local electric field to the applied average electric field is solely the field enhancement factor (Elocal = βEaverage). The value of β can be estimated from the slope (m) of the F–N plot, and quantifies the degree of enhancement in an electric field at the emission sites due to their nanometric dimension.37–54 In the present case, the field enhancement factor is calculated from the slope of the F–N plot (fitted linearly):
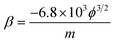 | (2) |
Along with the emission characteristics, current stability is one of the important parameters in the context of practical applications of cold cathodes. Fig. 3(c) depicts the emission current versus time (I–t) plot corresponding to preset values of ∼1 μA and ∼5 μA for the Ag NWs and rGO–Ag NWs, respectively, recorded over a period of 3 hours at a base pressure of 1 × 10−8 mbar. The emission current exhibits small excursions along with ‘spike’ type fluctuations superimposed on the base level. The average emission current is seen to remain extremely stable in the case of the rGO–Ag NWs as compared to the Ag NW emitter, which indicates good physical and chemical stability of the emitter. The inset of Fig. 3(c) shows a typical field emission micrograph of the hybrid rGO–Ag NW nanocomposite cathode, recorded at a current density of ∼150 μA cm−2.
Interestingly, it shows a large number of tiny bright spots, confirming that the emission is indeed from the most protruding Ag NWs and the atomically sharp edges of the rGO nanosheets. The overall superior field emission characteristics suggest that the hybrid rGO–Ag NW nanocomposite cathode can be considered as a promising electron source for practical applications in various vacuum micro-/nanoelectronic devices.
4. Conclusion
In conclusion, a polyol reflux technique was successfully used to synthesize rGO–Ag NW composites under optimized experimental conditions. Furthermore, the field emission properties of the Ag NWs, rGO and rGO–Ag NW nanocomposite have been investigated at a base pressure of ∼1 × 10−8 mbar. The turn-on field required to draw a current density of 1 μA cm−2 was found to be 5.0, 3.92 and 2.40 V μm−1 for the Ag NWs, rGO and the rGO–Ag NW composite, respectively. Enhanced field emission behaviour was observed for the rGO–Ag NW nanocomposite sample, due to its high field enhancement factor associated with the surface protrusions. In addition, the DFT results show that the enhanced field emission is due to overlapping in the electronic structures of the Ag NWs and rGO. The outstanding emission current density of the rGO–Ag NW composite at a low turn-on field and its nanometric structure morphology could be attractive for use as a suitable emitter for vacuum micro-/nanoelectronics and flat panel display device applications.Acknowledgements
Dr. D. J. Late would like to thank Prof. C. N. R. Rao (FRS), Director ICMS Bangalore (India), and Prof. Vinayak P. Dravid, Director NUANCE Centre (Northwestern University) USA, for encouragement and support. The research work was supported by the Department of Science and Technology (Government of India) through Ramanujan Fellowship to Dr. D. J. Late (grant no. SR/S2/RJN-130/2012) and Dr. C. S. Rout (grant no. SR/S2/RJN-21/2012), NCL-MLP project grant 028626, DST-SERB Fast-track Young scientist (project grant no. SB/FT/CS-116/2013, SB/FTP/PS-065/2013) and was partially supported by the INUP IITB project sponsored by DeitY, MCIT, Government of India and UGC-UKIERI thematic awards (grant no. UGC-2013-14/005). Aneeya K. Samantara acknowledges CSIR for fellowship. Dr. B. K. Jena acknowledges funding support from MNRE, New Delhi, India (no. 102/87/2011-NT), BRNS, Mumbai, India (no. 2013/37P/67/BRNS) and CSIR, New Delhi, India (Young Scientist Award Project YSP-2/2013, P-81-113). The authors are grateful to Prof. B. K. Mishra, Director CSIR-IMMT, for his kind permission and encouragement to do this study. RT thanks the SRM Research Institute, SRM University for providing supercomputing facilities and financial support. Mr. Sachin Suryawanshi gratefully acknowledges the financial support from BARC, Mumbai, for the award of Senior Research Fellowship under BARC-UoP memorandum (grant no. GOI-E-153). MAM would like to thank the BCUD, Savitribai Phule Pune University, India for the financial support provided for the field emission work under CNQS-UPE-UGC program activity. The authors thank Mr. A. Ghosh, IOP, Bhubaneswar for the SEM measurements.Notes and references
- Y. Sun and Y. Xia, Science, 2002, 298, 2176–2179 CrossRef CAS PubMed.
- H. Shiigi, Y. Yamamoto, N. Yoshi, H. Nakao and T. Nagaoka, Chem. Commun., 2006, 4288–4290 RSC.
- I. Pastoriza-Santos and L. M. Liz-Marzan, J. Mater. Chem., 2008, 18, 1724–1737 RSC.
- B. R. Martin, D. J. Dermody, B. D. Reiss, M. Fang, L. A. Lyon, M. J. Natan and T. E. Mallouk, Adv. Mater., 1999, 11, 1021–1025 CrossRef CAS.
- F. Kim, S. Connor, H. Song, T. Kuykendall and P. Yang, Angew. Chem., Int. Ed., 2004, 43, 3673–3677 CrossRef CAS PubMed.
- M. Grzelczak, J. Perez-Juste, P. Mulvaney and L. M. Liz-Marzan, Chem. Soc. Rev., 2008, 37, 1783–1791 RSC.
- Y. Sun, B. Gates, B. Mayers and Y. Xia, Nano Lett., 2002, 2, 165–168 CrossRef CAS.
- Y. Sun and Y. Xia, Adv. Mater., 2002, 14, 833–837 CrossRef CAS.
- H. Chen, L. Shao, Q. Li and J. Wang, Chem. Soc. Rev., 2013, 42, 2679–2724 RSC.
- M. A. El-Sayed, Acc. Chem. Res., 2001, 34, 257–264 CrossRef CAS PubMed.
- V. M. Cepak and C. R. Martin, J. Phys. Chem. B, 1998, 102, 9985–9990 CrossRef CAS.
- J. M. Renoirt, M. Debliquy, J. Albert, A. Ianoul and C. Caucheteur, J. Phys. Chem. C, 2014, 118, 11035–11042 CAS.
- R. F. Aroca, P. J. G. Goulet, D. S. D. Santos, R. A. Alvarez-Puebla and O. N. Oliveira, Anal. Chem., 2005, 77, 378–382 CrossRef CAS PubMed.
- Y. Cui, I. Y. Phang, R. S. Hegde, Y. H. Lee and X. Y. Ling, ACS Photonics, 2014, 1, 631–637 CrossRef CAS.
- J. W. Liu, J. L. Wang, W. R. Huang, L. Yu, X. F. Ren, W. C. Wen and S. H. Yu, Sci. Rep., 2012, 2, 987 Search PubMed.
- X. Liu, B. Wu, Q. Zhang, J. N. Yip, G. Yu, Q. Xiong, N. Mathews and T. C. Sum, ACS Nano, 2014, 8, 10101–10110 CrossRef CAS PubMed.
- X. Zhen, L. Zheng, H. Sun and C. Gao, Adv. Mater., 2013, 25, 3249–3253 CrossRef PubMed.
- R. Chen, S. R. Das, C. Jeong, M. R. Khan, D. B. Janes and M. A. Alam, Adv. Funct. Mater., 2013, 23, 5150–5158 CrossRef CAS PubMed.
- M. S. Lee, K. Lee, S. Y. Kim, H. Lee, J. Park, K. H. Choi, H. K. Kim, D. G. Kim, D. Y. Lee, S. W. Nam and J. U. Park, Nano Lett., 2013, 13, 2814–2821 CrossRef CAS PubMed.
- Y. J. Han, J. M. Kim and G. D. Stucky, Chem. Mater., 2000, 12, 2068–2069 CrossRef CAS.
- S. T. Hsiao, H. W. Tien, W. H. Liao, Y. S. Wang, S. M. Li, C. C. MMa, Y. H. Yu and W. P. Chuang, J. Mater. Chem. C, 2014, 2, 7284–7291 RSC.
- S. S. Kim, Y. R. Kim, T. D. Chung and B. H. Sohn, Adv. Funct. Mater., 2014, 24, 2764–2771 CrossRef CAS PubMed.
- Y. Ahn, Y. Jeong and Y. Lee, ACS Appl. Mater. Interfaces, 2012, 4, 6410–6414 CAS.
- S. C. Sahu, A. K. Samantara, A. Dash, R. R. Juluri, R. K. Sahu, B. K. Mishra and B. K. Jena, Nano Res., 2013, 6, 635–643 CrossRef CAS PubMed.
- S. C. Sahu, A. K. Samantara, B. Satpati, S. Bhattacharjee and B. K. Jena, Nanoscale, 2013, 5, 11265–11274 RSC.
- G. Park, L. Bartolome, K. G. Lee, S. J. Lee, D. H. Kim and T. J. Park, Nanoscale, 2012, 4, 3879–3885 RSC.
- G. Goncalves, P. A. A. P. Marques, C. M. Granadeiro, H. I. S. Nogueira, M. K. Singh and J. Grácio, Chem. Mater., 2009, 21, 4796–4802 CrossRef CAS.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- L. Hu, H. S. Kim, J. Y. Lee, P. Peumans and Y. Cui, ACS Nano, 2010, 4, 2955–2963 CrossRef CAS PubMed.
- G. Kresse and J. J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS.
- B. Das, R. Voggu, C. S. Rout and C. N. R. Rao, Chem. Commun., 2008, 5155–5157 RSC.
- G. Fursey, Field emission in vacuum microelectronics, Kluwer Academic/Plenum publishers, New York, 2005 Search PubMed.
- R. H. Fowler and L. Nordheim, Proc. R. Soc. London, 1928, 119, 173–181 CrossRef CAS.
- C. S. Rout, P. D. Joshi, R. V. Kashid, D. S. Joag, M. A. More, A. J. Simbeck, M. Washington, S. K. Nayak and D. J. Late, Appl. Phys. Lett., 2014, 105, 043109 CrossRef PubMed.
- C. S. Rout, P. D. Joshi, R. V. Kashid, D. S. Joag, M. A. More, A. J. Simbeck, M. Washington, S. K. Nayak and D. J. Late, Sci. Rep., 2013, 3, 3282 Search PubMed.
- C. Jiang, S. Liu, X. Chen and S. Yu, CrystEngComm, 2014, 16, 8646–8651 RSC.
- R. R. Devarapalli, D. R. Shinde, F. Barka-Bouaifel, S. G. Yenchalwar, R. Boukherroub, M. A. More and M. V. Shelke, J. Mater. Chem., 2012, 22, 22922 RSC.
- C. S. Rout, R. Tiwari, R. V. Kashid, D. S. Joag, M. A. More, A. J. Simbeck, M. Washington, S. K. Nayak and D. J. Late, Eur. J. Inorg. Chem., 2014,(31), 5331–5336 CrossRef CAS PubMed.
- R. V. Kashid, D. J. Late, S. S. Chou, Y. Huang, M. De, D. S. Joag, M. A. More and V. P. Dravid, Small, 2013, 9, 2730–2734 CrossRef CAS PubMed.
- B. A. Kakade, V. K. Pillai, D. J. Late, P. G. Chavan, F. J. Sheini, M. A. More and D. S. Joag, Appl. Phys. Lett., 2010, 97, 073102 CrossRef PubMed.
- D. J. Late, P. Misra, B. N. Singh, L. M. Kukreja, D. S. Joag and M. A. More, Appl. Phys. A: Mater. Sci. Process., 2009, 95, 613–620 CrossRef CAS PubMed.
- S. R. Suryawanshi, P. S. Kolhe, C. S. Rout, D. J. Late and M. A. More, Ultramicroscopy, 2015, 149, 51–57 CrossRef CAS PubMed.
- D. J. Late, M. A. More, D. S. Joag, P. Misra, B. N. Singh and L. M. Kukreja, Appl. Phys. Lett., 2006, 89, 123510 CrossRef PubMed.
- D. J. Late, M. A. More, P. Misra, B. N. Singh, L. M. Kukreja and D. S. Joag, Ultramicroscopy, 2007, 107, 825–832 CrossRef CAS PubMed.
- D. J. Late, K. S. Date, M. A. More, P. Misra, B. N. Singh, L. M. Kukreja, C. V. Dharmadhikari and D. S. Joag, Appl. Surf. Sci., 2008, 254, 3601–3605 CrossRef CAS PubMed.
- D. S. Joag, D. J. Late and U. D. Lanke, Solid State Commun., 2004, 130, 305–308 CrossRef CAS PubMed.
- R. B. Sharma, D. J. Late, D. S. Joag, A. Govindaraj and C. N. R. Rao, Chem. Phys. Lett., 2006, 428, 102–108 CrossRef CAS PubMed.
- R. Khare, D. B. Shinde, S. Bansode, M. A. More, M. Majumder, V. K. Pillai and D. J. Late, Appl. Phys. Lett., 2015, 106, 023111 CrossRef PubMed.
- S. Ratha, R. Khare, M. A. More, R. Thapa, D. J. Late and C. S. Rout, RSC Adv., 2015, 5, 5372–5378 RSC.
- D. J. Late, P. A. Shaikh, R. Khare, R. V. Kashid, M. Chaudhary, M. A. More and S. B. Ogale, ACS Appl. Mater. Interfaces, 2014, 6, 15881–15888 CAS.
- K. K. Naik, R. Khare, D. Chakravarty, M. A. More, R. Thapa, D. J. Late and C. S. Rout, Appl. Phys. Lett., 2014, 105, 233101 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: FESEM, TEM and XRD characterization of GO and rGO. See DOI: 10.1039/c5ra00308c |
| This journal is © The Royal Society of Chemistry 2015 |