Lithium metatitanate enhanced solid–solid reaction in a lithium–nitrogen–hydrogen system
Abstract
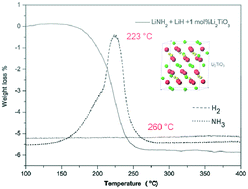
We have decreased the end temperature of the Li–N–H system, a hydrogen storage material developed in 2002, to below 260 °C, and obtained a lowest peak temperature of 223 °C.

 Please wait while we load your content...
Please wait while we load your content...