Temperature induced gelation transition of a fumed silica/PEG shear thickening fluid
Abstract
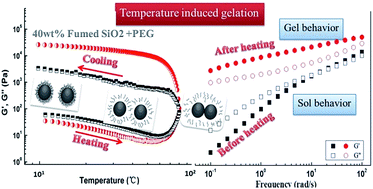
The effect of temperature on the rheological behaviors of a shear thickening fluid (STF) prepared by dispersing fumed silica (SiO2) particles into polyethylene glycol (PEG) under mechanical stirring and ultrasonication was investigated using a rotational rheometer. Under steady shear, the system showed an obvious shear thickening behavior due to the formation of “hydroclusters” of SiO2 particles driven by hydrodynamic lubrication forces. The value of the critical shear rate at which the shear thickening begins grows monotonically with temperature. Dynamic temperature sweeps show that elevating the temperature induces a gelation transition of the SiO2/PEG system when the concentration of SiO2 exceeds a critical value, which is found to be lower for the system consisting of higher average molecular weight PEG. The gelation process also becomes more remarkable at a higher concentration of SiO2 particles. It is found that the temperature induced gelation of SiO2/PEG sol is essentially related to the disappearance of the solvation layer on the surface of SiO2 particles as well as the change of hydrogen bonds.

 Please wait while we load your content...
Please wait while we load your content...