Synthesis of poly(stearyl methacrylate-b-3-phenylpropyl methacrylate) nanoparticles in n-octane and associated thermoreversible polymorphism
Abstract
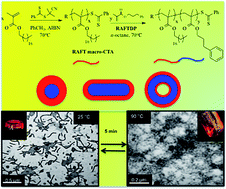
Poly(stearyl methacrylate) (PSMA) homopolymers with average degrees of polymerization (![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif) n) ranging from 18–30 have been prepared by homogeneous RAFT radical polymerization in toluene and subsequently employed as macro-chain transfer agents (CTAs) in non-polar RAFT dispersion formulations with 3-phenylpropyl methacrylate (PPMA) as the comonomer in n-octane at 70 °C. With PSMA18 or PSMA19 macro-CTAs in n-octane at 20 wt%, a series of PSMAx–PPPMAy block copolymers are readily accessible in situ that form the full range of common nanoparticle morphologies, with the complexity of the nano-objects increasing (spheres-to-worms-to-vesicles) with increasing
n) ranging from 18–30 have been prepared by homogeneous RAFT radical polymerization in toluene and subsequently employed as macro-chain transfer agents (CTAs) in non-polar RAFT dispersion formulations with 3-phenylpropyl methacrylate (PPMA) as the comonomer in n-octane at 70 °C. With PSMA18 or PSMA19 macro-CTAs in n-octane at 20 wt%, a series of PSMAx–PPPMAy block copolymers are readily accessible in situ that form the full range of common nanoparticle morphologies, with the complexity of the nano-objects increasing (spheres-to-worms-to-vesicles) with increasing ![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif) n of the PPPMA block as clearly evidenced by transmission electron microscopy (TEM). An evaluation of the effect of total solids for the preparation of block copolymers of common composition indicated that polymerizations conducted at higher concentrations favoured the formation of nanoparticles with more complex morphologies. In the case of block copolymers prepared with a PSMA30 macro-CTA the only accessible morphology was spheres regardless of compositional asymmetry. However, the size of the spheres increased monotonically with increasing PPPMA block length. Formulations that yielded (essentially) pure worm phases, such as PSMA18-b-PPPMA71, formed physical gels at ambient temperature. Heating the physical gels to (or beyond) a critical temperature resulted in a macroscopic transformation to a free flowing solution. The fundamental reason for the transformation, as evidenced by TEM, was a morphological transition from worm to sphere nanoparticles facilitated, in part, by a change in solvation of the PPPMA core-forming block with increasing temperature. DLS analysis indicated that the morphology transitions were fully reversible.
n of the PPPMA block as clearly evidenced by transmission electron microscopy (TEM). An evaluation of the effect of total solids for the preparation of block copolymers of common composition indicated that polymerizations conducted at higher concentrations favoured the formation of nanoparticles with more complex morphologies. In the case of block copolymers prepared with a PSMA30 macro-CTA the only accessible morphology was spheres regardless of compositional asymmetry. However, the size of the spheres increased monotonically with increasing PPPMA block length. Formulations that yielded (essentially) pure worm phases, such as PSMA18-b-PPPMA71, formed physical gels at ambient temperature. Heating the physical gels to (or beyond) a critical temperature resulted in a macroscopic transformation to a free flowing solution. The fundamental reason for the transformation, as evidenced by TEM, was a morphological transition from worm to sphere nanoparticles facilitated, in part, by a change in solvation of the PPPMA core-forming block with increasing temperature. DLS analysis indicated that the morphology transitions were fully reversible.

 Please wait while we load your content...
Please wait while we load your content...