Potato starch: binder and pore former in nanoframes of nanolayered oxides for Pb2+ and Ni2+ as pollutants in water and industrial sludge applications
Abstract
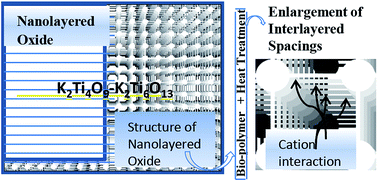
This article deals with the importance of potato starch in two different applications, as a pore former and also as a binder in microporous/mesoporous ceramic adsorbents for the removal of toxic metal pollutants in water. These porous ceramic adsorbents have unique and high adsorption and ion exchange properties in addition to an intricate structure, and were obtained by mixing 50% potassium polytitanates (PPTs) and 50% potato starch (PS). Cylindrical pieces were obtained through a typical extrusion process. Additionally, a heat treatment in different stages was applied to these cylinders and later they were crushed to 2–5 mm size. The physicochemical behavior of the adsorbents was assessed before and after the sintering process using different techniques: thermal analysis, chemical analysis, optical microscopy, SEM, TEM, XRD, BET, mercury porosimetry, determination of mechanical properties and physicochemical methods. Amounts of PS of 5–50 weight percent were applied with PPT in order to produce adsorbents with a properly configured structure. The mechanical properties and results of adsorption tests showed that the adsorbents removed cations of lead and nickel from the solution excellently. PPTs have technological and academic importance attributed to the fact that they have not yet been studied in linked form. It is possible to reduce diseases in people across the five continents by treatment of polluted water using these materials, and they also provide an opportunity for usage of potato waste.

 Please wait while we load your content...
Please wait while we load your content...