Tunable green to red ZrO2:Er nanophosphors
Abstract
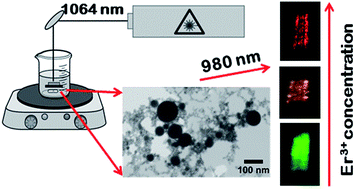
Pulsed laser ablation in water was validated as an effective method to produce highly crystalline erbium doped ZrO2 nanoparticles. Different concentrations of erbium doped ZrO2 ceramic precursor targets were used in the ablation, to study the efficiency of erbium incorporation in the zirconia lattice during nanoparticle synthesis by this method. The spherical nanoparticles produced, with a diameter of up to 200 nm, preserve the crystallinity and optical properties of the precursor target, even for the higher dopant amounts. The optical activation of Er3+ ions was achieved without the need for any additional thermal annealing, usually required for particles produced by several chemical routes. A tunable green to red color of the ZrO2:Er3+ nanoparticles is accomplished through the manipulation of the erbium ion concentration. Particularly, through the sequential absorption of two infrared photons, intense visible up conversion luminescence was observed at room temperature highlighting the doped nanoparticles as promising alternative imaging agents.

 Please wait while we load your content...
Please wait while we load your content...