TiO2 immobilized zein microspheres: a biocompatible adsorbent for effective dye decolourisation
Abstract
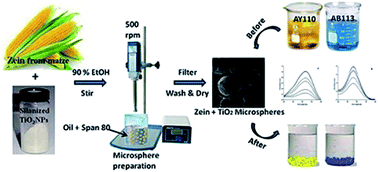
Natural protein polymers and metal oxide nanoparticles individually possess potential efficiency for dye binding and photocatalytic dye degradation, respectively. To attain these multifunctional properties, we have developed a composite zein–TiO2 microsphere, characterized it and evaluated its dye adsorption and photocatalytic efficiency. The phase structure, morphology, elemental composition and porous nature of the microspheres were characterized by using X-ray diffraction (XRD), Fourier transform-infra red (FTIR) spectroscopy, field emission-scanning electron microscopy (FE-SEM) with energy dispersive X-ray spectroscopy (EDAX), and nitrogen adsorption–desorption isotherm measurements. Adsorption experiments were performed by varying different parameters like contact time, initial dye concentration and adsorbent dosage, and the various adsorption isotherms and kinetics were tested. The results revealed that the Langmuir and Tempkin isotherms were more appropriate and followed a pseudo-first order model for the adsorption of acid yellow (AY110) and acid blue (AB113) dyes. The fluorescence probe method was used to confirm the generation of ˙OH radicals and elucidated the photodegradation mechanism. Therefore, metallo-polymer based adsorbents provide new scope in the selection of ideal adsorbents for efficient separation and remediation technologies in tannery effluent management.

 Please wait while we load your content...
Please wait while we load your content...