Bis(carbamoylmethylphosphine oxide) ligands fixed on the arene core via 1,2,3-triazole linkers: novel effective extractants for palladium, lanthanides and actinides
Alexander N. Turanova,
Vasilii K. Karandashev*bc,
Elena V. Sharovad,
Galina K. Genkinad and
Oleg I. Artyushind
aInstitute of Solid State Physics, Russian Academy of Sciences, 142432 Chernogolovka, Russia
bInstitute of Microelectronics Technology and High Purity Materials, Russian Academy of Sciences, 142432 Chernogolovka, Russia
cNational University of Science and Technology MISiS, 119049, Moscow, Russia. E-mail: karan@iptm.ru
dA. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 119991 Moscow, Russia
First published on 12th March 2015
Abstract
Novel polydentate neutral organophosphorus ligands containing two diphenyl-(diethylcarbamoylmethyl)phosphine oxide moieties fixed on the arene platform via methylene-1,2,3-triazole linkers (Ar-CMPO) were synthesized by the method of “click” chemistry and studied as an extractant for Pd(II), U(VI), Th(IV) and lanthanide(III) ions from HNO3 solutions. The influence of aqueous and organic phases on the extraction efficiency was elucidated and the stoichiometry of the complexes extracted was determined. The introduction of a 1,2,3-triazole fragment into the methylene bridge of a CMPO molecule resulted in a considerable increase in the efficiency of Pd(II) extraction. This modification of a CMPO molecule does not essentially change the extraction efficiency towards U(VI) and Ln(III) but drastically decreases the Th(IV) extraction and changes the order in the extractability of U(VI) and Th(IV) from HNO3 solutions. Bis-CMPO ligands containing two 1,2,3-triazole moieties (Ar-CMPO) were found to possess a higher extraction efficiency towards Pd(II), U(VI), Th(IV) and lanthanide(III) ions than their mono analog 2-(diphenylphosphoryl)-N,N-diethyl-3-(1-phenyl-1H-1,2,3-triazol-4-yl)propanamide.
Introduction
Solvent extraction is a widely used technique for the recovery and separation of actinides and lanthanides from high-level liquid waste (HLLW) of nuclear fuel cycles. HLLW can be a valuable source of platinum group metals. The recovery of fission palladium is of particular interest and is being investigated world-wide.1 Bidentate neutral organophosphorus compounds such as diaryl- or alkyl(aryl)[dialkylcarbamoylmethyl]phosphine oxides (CMPO) are known to be efficient extractants for the extraction of hard actinide and lanthanide ions from nitric acid solutions.2,3 However, these compounds do not show any appreciable ability to extract soft Pd(II) ions from nitric acid solutions.1 A difference in extractabilities of Pd(II) and Ln(III) ions with polyfunctional neutral extractants depends on the nature of coordinating moieties of an extractant molecule. It is known that the diamide extractant tetraoctyldiglycolamide (TODGA) with hard oxygen donors extract lanthanides and actinides from nitric acid solutions more strongly than Pd(II).4 Replacement of the central ether oxygen in the molecule of diamides of diglycolic acid by soft nitrogen donors changes the relation between the extractabilities of lanthanides and Pd(II). Sasaki et al. showed that 2,2′-(methylimino)bis(N,N-dioctylacetamide), which has a frame similar to that of TODGA and a nitrogen atom instead of the ether oxygen, effectively extracts Pd(II) from nitric acid solutions whereas the extractability of Am(III) and lanthanides is low.5Recently, we have found that the introduction of a 1,2,3-triazole fragment into the methylene bridge of a CMPO molecule resulted in a considerable increase of the efficiency of Pd(II) extraction.6 However, the extraction efficiency of 2-(diphenylphosphoryl)-N,N-diethyl-3-(1-phenyl-1H-1,2,3-triazol-4-yl)propanamide towards Ln(III) slightly decreased as compared with that of unsubstituted CMPO.6 It is known that the efficiency and selectivity of CMPO type ligands usually increase with an increase in the amount of CMPO residues in a molecule.7,8 Attachment of CMPO groups to molecular platforms such as calixarenes9–11 and cavitands12–15 led to further increase of extraction efficiencies and selectivities. Therefore, it seemed reasonable to attempt the synthesis of novel polyfunctional neutral organophosphorus compounds Ar-CMPO 1–7 and estimate their extraction properties towards Pd(II), U(VI), Th(IV) and lanthanides(III). This study was carried as part of the research on the specific features of the extraction ability of polyfunctional neutral organophosphorus compounds, depending on the variation in their molecule structure. In this report we describe the results of our studies on some extraction properties of Ar-CMPO 1–7 compounds towards Pd(II), U(VI), Th(IV) and lanthanides(III) in nitric acid media. The extraction behavior of these compounds is compared with that of its structural mono analog 8 (Scheme 1).
Experimental
The NMR spectra were recorded on a Bruker AMX-400 instrument in CDCl3 solution. The chemical shifts (δ) were internally referenced by the residual solvent signals relative to tetramethylsilane (1H and 13C) or externally to H3PO4 (31P). The 13C NMR spectra were registered using the JMODECHO mode; the signals for the C atoms bearing odd and even numbers of protons have opposite polarities. IR spectra were recorded on a Magna-IR 750 FTIR spectrometer (Nicolet Co., resolution 2 cm−1, scan number 128, KBr pellets). Melting points were determined with an Electrothermal IA9100 Digital Melting Point Apparatus and were uncorrected. Chemical and analytical grade hexane, acetone, 1,2-dichloroethane, tert-butanol and methanol were used as diluents.The ligand 6 (ref. 6) was obtained by coupling a phenylazide with 2-propargyl-diphenyl(N,N-diethylcarbamoyl-methyl)phosphine oxide by procedure of “click” chemistry. The synthesis of compound 8 (ref. 16) was described previously.
Synthesis of the ligands
The desired polydentate neutral organophosphorus ligands 1–6 were synthesized using the general synthetic procedure of “click” chemistry detailed by us earlier for compound 7, namely by copper(I)-catalyzed 1,3-dipolar cycloaddition of corresponding diazides 1a–6a to 2-propargyl-diphenyl(N,N-diethyl-carbamoylmethyl)phosphine oxide,6 according to the general route deplicated in the Scheme 2.The starting diazides 1a,17 2a and 3a,18 4a,19 5a,20 and 6a21 were obtained via the procedures described in the literature, and their physicochemical constants matched the literature data.
A general procedure for the preparation of 1–6
To a solution of 2-propargyl-diphenyl(N,N-diethylcarbamoyl-methyl)phosphine oxide (0.5 g, 1.35 mmol) and corresponding diazide 1a–6a in t-BuOH–H2O (5 mL) in the ratio 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was added CuSO4·5H2O (5 mol%, 17 mg, 0.0675 mmol) and sodium ascorbate (10 mol%, 27 mg, 0.135 mmol) with stirring. The mixture was stirred at room temperature for 60–70 h. The reaction was completed as verified by TLC and 31P NMR control. Then, the solvent was evaporated under reduced pressure and CH2Cl2 was added to the residue (30 mL). The product was washed with 3% aq. NH3 (3 × 15 mL). The collected organic layers were dried over Na2SO4, filtered and the solvent was evaporated in vacuo. The residue was purified by thin-layer column chromatography (SiO2) using hexane–acetone (from 100
2 was added CuSO4·5H2O (5 mol%, 17 mg, 0.0675 mmol) and sodium ascorbate (10 mol%, 27 mg, 0.135 mmol) with stirring. The mixture was stirred at room temperature for 60–70 h. The reaction was completed as verified by TLC and 31P NMR control. Then, the solvent was evaporated under reduced pressure and CH2Cl2 was added to the residue (30 mL). The product was washed with 3% aq. NH3 (3 × 15 mL). The collected organic layers were dried over Na2SO4, filtered and the solvent was evaporated in vacuo. The residue was purified by thin-layer column chromatography (SiO2) using hexane–acetone (from 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 to 10
20 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, v/v) and then CH2Cl2–methanol (100
100, v/v) and then CH2Cl2–methanol (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v). The identity and the purity of the reaction products were established by their spectral (1H, 31P, 13C NMR, IR) data.
10, v/v). The identity and the purity of the reaction products were established by their spectral (1H, 31P, 13C NMR, IR) data.
Note that isolated ligands after careful drying over P2O5 in vacuum contained 1 or 1.5 mol of water according to the 1H NMR, IR and elemental analysis data. The numeration of the central arene core in Ar-CMPO 1–6 is presented in the figures (vide infra).
Yield: 60%. Mp: 189–193 °C. IR (KBr, cm−1): 3431 (νOH), 2969, 2929, 1631 (νC
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1460 (νAr), 1437, 1215 (νP
O), 1460 (νAr), 1437, 1215 (νP![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1190, 1118, 1099, 705, 516. 1H NMR (400 MHz, CDCl3, ppm, J/Hz): 0.72 and 0.75 (both t, 6H + 6H, NCH2CH3, 3JHH = 7.2); 2.78 and 2.96 (both dd, 2H + 2H, CH2CH(P(O)Ph2)–, 3JHH = 11.1, 3JPH = 13.0); 3.10–3.45 (m, 8H, NCH2CH3); 4.19 (ddd, 2H, PCH, 2JPH = 14.8, 3JHH = 11.1); 5.48 (br. s, 4H, –CH2–Ar–CH2–); 7.03–7.08 (m, 2H, H(C4) + H(C5) in Ar); 7.23 (br. s, 2H, CH in triazole cycle); 7.26–7.32 (m, 2H, H(C3) + H(C6)); 7.42–7.60, 7.83–7.92, 8.05–8.15 (all m, 12H + 4H + 4H, C6H5–P). 31P NMR (161.97 MHz, CDCl3): 30.11 ppm. 13C NMR (100.61 MHz, CDCl3, ppm, J/Hz): 12.42 and 13.76 (both s, CH3CH2N), 23.30 (br. s, CH2CH(P(O)Ph2–), 40.85 and 42.49 (both s, CH3CH2N), 45.76 (d, P–CH, 1JPC = 60.9), 50.78 (s, –CH2–Ar–CH2–), 122.24 (s,
O), 1190, 1118, 1099, 705, 516. 1H NMR (400 MHz, CDCl3, ppm, J/Hz): 0.72 and 0.75 (both t, 6H + 6H, NCH2CH3, 3JHH = 7.2); 2.78 and 2.96 (both dd, 2H + 2H, CH2CH(P(O)Ph2)–, 3JHH = 11.1, 3JPH = 13.0); 3.10–3.45 (m, 8H, NCH2CH3); 4.19 (ddd, 2H, PCH, 2JPH = 14.8, 3JHH = 11.1); 5.48 (br. s, 4H, –CH2–Ar–CH2–); 7.03–7.08 (m, 2H, H(C4) + H(C5) in Ar); 7.23 (br. s, 2H, CH in triazole cycle); 7.26–7.32 (m, 2H, H(C3) + H(C6)); 7.42–7.60, 7.83–7.92, 8.05–8.15 (all m, 12H + 4H + 4H, C6H5–P). 31P NMR (161.97 MHz, CDCl3): 30.11 ppm. 13C NMR (100.61 MHz, CDCl3, ppm, J/Hz): 12.42 and 13.76 (both s, CH3CH2N), 23.30 (br. s, CH2CH(P(O)Ph2–), 40.85 and 42.49 (both s, CH3CH2N), 45.76 (d, P–CH, 1JPC = 60.9), 50.78 (s, –CH2–Ar–CH2–), 122.24 (s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– in triazole cycle), 128.29 and 128.46 (both d, o-C in C6H5, 2JPC = 11.7), 129.40 (s, C3, C6 in Ar), 129.81 (s, C4, C5 in Ar), 130.68 and 130.77 (both d, ipso-C in C6H5, 1JPC = 99.4), 131.67 and 131.84 (both d, m-C in C6H5, 3JPC = 9.2), 131.94 and 132.02 (both d, p-C in C6H5, 4JPC = 2.6), 133.27 (s, ipso-C1,C2 in Ar), 144.98 and 145.00 (both d, –C
CH– in triazole cycle), 128.29 and 128.46 (both d, o-C in C6H5, 2JPC = 11.7), 129.40 (s, C3, C6 in Ar), 129.81 (s, C4, C5 in Ar), 130.68 and 130.77 (both d, ipso-C in C6H5, 1JPC = 99.4), 131.67 and 131.84 (both d, m-C in C6H5, 3JPC = 9.2), 131.94 and 132.02 (both d, p-C in C6H5, 4JPC = 2.6), 133.27 (s, ipso-C1,C2 in Ar), 144.98 and 145.00 (both d, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– in triazole cycle, 3JPC = 15.5), 167.20 (s, C
CH– in triazole cycle, 3JPC = 15.5), 167.20 (s, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). Anal. calcd for C50H56N8O4P2·1.5H2O: C, 65.13; H, 6.91; N, 12.15; P, 6.72. Found: C, 65.32; H, 6.78; N, 12.38; P, 6.54.
O). Anal. calcd for C50H56N8O4P2·1.5H2O: C, 65.13; H, 6.91; N, 12.15; P, 6.72. Found: C, 65.32; H, 6.78; N, 12.38; P, 6.54.Yield: 57%. Mp: 180–183 °C. IR (KBr, cm−1): 3436 (νOH), 2974, 2932, 1630 (νC
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1461 (νAr), 1437, 1190 (νP
O), 1461 (νAr), 1437, 1190 (νP![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1117, 1099, 728, 705, 516. 1H NMR (400 MHz, CDCl3, ppm, J/Hz): 0.50 and 0.54 (both t, 6H + 6H, NCH2CH3, 3JHH = 7.1); 2.53–2.63, 2.73–2.83, 2.90–3.06, 3.15–3.27 (all m, 12H, NCH2CH3 + CH2CH(P(O)Ph2)–); 4.02 (ddd, 2H, PCH, 2JPH = 13.4, 3JHH = 11.1); 5.21 (br. s, 4H, –CH2–Ar–CH2–); 6.93 (d, 2H, H(C4) + H(C6) in Ar, 3JHH = 7.6); 7.03 (br. s, 2H, CH in triazole cycle); 7.07 (t, 1H, H(C5), 3JHH = 7.6); 7.14 (s, 1H, H(C2)); 7.25–7.43, 7.65–7.75, 7.84–7.95 (all m, 12H + 4H + 4H, C6H5–P). 31P NMR (161.97 MHz, CDCl3): 30.06 ppm. 13C NMR (100.61 MHz, CDCl3, ppm, J/Hz): 11.97 and 13.31 (both s, CH3CH2N), 24.14 (s, CH2CH(P(O)Ph2–), 40.32 and 42.06 (both s, CH3CH2N), 44.63 (d, P–CH, 1JPC = 60.9), 52.92 (s, –CH2–Ar–CH2–), 121.87 (s,
O), 1117, 1099, 728, 705, 516. 1H NMR (400 MHz, CDCl3, ppm, J/Hz): 0.50 and 0.54 (both t, 6H + 6H, NCH2CH3, 3JHH = 7.1); 2.53–2.63, 2.73–2.83, 2.90–3.06, 3.15–3.27 (all m, 12H, NCH2CH3 + CH2CH(P(O)Ph2)–); 4.02 (ddd, 2H, PCH, 2JPH = 13.4, 3JHH = 11.1); 5.21 (br. s, 4H, –CH2–Ar–CH2–); 6.93 (d, 2H, H(C4) + H(C6) in Ar, 3JHH = 7.6); 7.03 (br. s, 2H, CH in triazole cycle); 7.07 (t, 1H, H(C5), 3JHH = 7.6); 7.14 (s, 1H, H(C2)); 7.25–7.43, 7.65–7.75, 7.84–7.95 (all m, 12H + 4H + 4H, C6H5–P). 31P NMR (161.97 MHz, CDCl3): 30.06 ppm. 13C NMR (100.61 MHz, CDCl3, ppm, J/Hz): 11.97 and 13.31 (both s, CH3CH2N), 24.14 (s, CH2CH(P(O)Ph2–), 40.32 and 42.06 (both s, CH3CH2N), 44.63 (d, P–CH, 1JPC = 60.9), 52.92 (s, –CH2–Ar–CH2–), 121.87 (s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– in triazole cycle), 127.19 (s, C2 in Ar), 127.53 (s, C4, C6 in Ar), 128.00 and 128.07 (both d, o-C in C6H5, 2JPC = 12.1), 129.14 (s, C5 in Ar), 130.14 and 130.30 (both d, ipso-C in C6H5, 1JPC = 99.0), 131.16 and 131.38 (both d, m-C in C6H5, 3JPC = 9.5), 131.69 and 131.70 (both d, p-C in C6H5, 4JPC = 2.0), 135.26 (s, ipso-C1, C3 in Ar), 144.41 (d, –C
CH– in triazole cycle), 127.19 (s, C2 in Ar), 127.53 (s, C4, C6 in Ar), 128.00 and 128.07 (both d, o-C in C6H5, 2JPC = 12.1), 129.14 (s, C5 in Ar), 130.14 and 130.30 (both d, ipso-C in C6H5, 1JPC = 99.0), 131.16 and 131.38 (both d, m-C in C6H5, 3JPC = 9.5), 131.69 and 131.70 (both d, p-C in C6H5, 4JPC = 2.0), 135.26 (s, ipso-C1, C3 in Ar), 144.41 (d, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– in triazole cycle, 3JPC = 15.7), 166.79 (s, C
CH– in triazole cycle, 3JPC = 15.7), 166.79 (s, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). Anal. calcd for C50H56N8O4P2·1.5H2O: C, 65.13; H, 6.91; N, 12.15; P, 6.72. Found: C, 65.25; H, 6.98; N, 12.10; P, 6.65.
O). Anal. calcd for C50H56N8O4P2·1.5H2O: C, 65.13; H, 6.91; N, 12.15; P, 6.72. Found: C, 65.25; H, 6.98; N, 12.10; P, 6.65.Yield: 50%. Mp: 195–198 °C. IR (KBr, cm−1): 3436 (νOH), 2973, 2931, 1630 (νC
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1461 (νAr), 1437, 1217 (νP
O), 1461 (νAr), 1437, 1217 (νP![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1188, 1099, 705, 516. 1H NMR (400 MHz, CDCl3, ppm, J/Hz): 0.71 and 0.76 (both t, 6H + 6H, NCH2CH3, 3JHH = 7.2); 2.75–2.87, 2.93–3.08, 3.10–3.30, 3.32–3.48 (all m, 12H, NCH2CH3 + CH2CH(P(O)Ph2)–); 4.21 (ddd, 2H, PCH, 2JPH = 13.4, 3JHH = 11.5); 5.41 (br. s, 4H, –CH2–Ar–CH2–); 7.18 (br. s, 4H, H(C2) + H(C3) + H(C5) + H(C6) in Ar); 7.24 (br. s, 2H, CH in triazole cycle); 7.45–7.65, 7.85–7.96, 8.07–8.18 (all m, 12H + 4H + 4H, C6H5–P). 31P NMR (161.97 MHz, CDCl3): 30.09 ppm. 13C NMR (100.61 MHz, CDCl3, ppm, J/Hz): 12.41 and 13.75 (both s, CH3CH2N), 24.65 (s, CH2CH(P(O)Ph2–), 40.79 and 42.52 (both s, CH3CH2N), 45.65 (d, P–CH, 1JPC = 60.1), 53.26 (br. s, –CH2–Ar–CH2–), 122.03 (s,
O), 1188, 1099, 705, 516. 1H NMR (400 MHz, CDCl3, ppm, J/Hz): 0.71 and 0.76 (both t, 6H + 6H, NCH2CH3, 3JHH = 7.2); 2.75–2.87, 2.93–3.08, 3.10–3.30, 3.32–3.48 (all m, 12H, NCH2CH3 + CH2CH(P(O)Ph2)–); 4.21 (ddd, 2H, PCH, 2JPH = 13.4, 3JHH = 11.5); 5.41 (br. s, 4H, –CH2–Ar–CH2–); 7.18 (br. s, 4H, H(C2) + H(C3) + H(C5) + H(C6) in Ar); 7.24 (br. s, 2H, CH in triazole cycle); 7.45–7.65, 7.85–7.96, 8.07–8.18 (all m, 12H + 4H + 4H, C6H5–P). 31P NMR (161.97 MHz, CDCl3): 30.09 ppm. 13C NMR (100.61 MHz, CDCl3, ppm, J/Hz): 12.41 and 13.75 (both s, CH3CH2N), 24.65 (s, CH2CH(P(O)Ph2–), 40.79 and 42.52 (both s, CH3CH2N), 45.65 (d, P–CH, 1JPC = 60.1), 53.26 (br. s, –CH2–Ar–CH2–), 122.03 (s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– in triazole cycle), 124.90 (s, C2, C3, C5, C6 in Ar), 128.33 and 128.47 (both d, o-C in C6H5, 2JPC = 13.9), 130.81 (d, ipso-C in C6H5, 1JPC = 99.4), 131.69 and 131.87 (both d, m-C in C6H5, 3JPC = 9.2), 131.94 and 132.01 (both d, p-C in C6H5, 4JPC = 3.6), 135.20 (s, ipso-C1, C4 in Ar), 145.03 (d, –C
CH– in triazole cycle), 124.90 (s, C2, C3, C5, C6 in Ar), 128.33 and 128.47 (both d, o-C in C6H5, 2JPC = 13.9), 130.81 (d, ipso-C in C6H5, 1JPC = 99.4), 131.69 and 131.87 (both d, m-C in C6H5, 3JPC = 9.2), 131.94 and 132.01 (both d, p-C in C6H5, 4JPC = 3.6), 135.20 (s, ipso-C1, C4 in Ar), 145.03 (d, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– in triazole cycle, 3JPC = 15.4), 167.24 (s, C
CH– in triazole cycle, 3JPC = 15.4), 167.24 (s, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). Anal. calcd for C50H56N8O4P2·1.5H2O: C, 65.13; H, 6.91; N, 12.15; P, 6.72. Found: C, 65.40; H, 6.90; N, 12.32; P, 6.83.
O). Anal. calcd for C50H56N8O4P2·1.5H2O: C, 65.13; H, 6.91; N, 12.15; P, 6.72. Found: C, 65.40; H, 6.90; N, 12.32; P, 6.83.Yield: 62%. Mp: 110–115 °C. IR (KBr, cm−1): 3436 (νOH), 2974, 2932, 1630 (νC
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1461 (νAr), 1437, 1188 (νP
O), 1461 (νAr), 1437, 1188 (νP![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1117, 1099, 704, 515. 1H NMR (400 MHz, CDCl3, ppm, J/Hz): 0.70 and 0.72 (both t, 6H + 6H, NCH2CH3, 3JHH = 7.6); 2.70–2.82, 2.93–3.03, 3.06–3.25, 3.33–3.42 (all m, 12H, NCH2CH3 + CH2CH(P(O)Ph2)–); 4.17 (ddd, 2H, PCH, 2JPH = 13.5, 3JHH = 11.1); 5.45 (dd, 4H, –CH2–Ar–CH2, 2JHH = 14.9, 2JHH = 18.0); 7.21 and 7.44 (both d, 4H + 4H, H(C2) + H(C3) + H(C6) + H(C7) + H(C9) + H(C10) + H(C11) + H(C12) in Ar, 3JHH = 8.4); 7.28 (br. s, 2H, CH in triazole cycle); 7.45–7.55, 7.84–7.90, 8.06–8.12 (all m, 12H + 4H + 4H, C6H5–P). 31P NMR (161.97 MHz, CDCl3): 29.95 ppm. 13C NMR (100.61 MHz, CDCl3, ppm, J/Hz): 12.44 and 13.79 (both s, CH3CH2N), 24.75 (s, CH2CH(P(O)Ph2–), 40.83 and 42.55 (both s, CH3CH2N), 45.79 (d, P–CH, 1JPC = 60.9), 53.23 (br. s, –CH2–Ar–CH2–), 121.95 (s,
O), 1117, 1099, 704, 515. 1H NMR (400 MHz, CDCl3, ppm, J/Hz): 0.70 and 0.72 (both t, 6H + 6H, NCH2CH3, 3JHH = 7.6); 2.70–2.82, 2.93–3.03, 3.06–3.25, 3.33–3.42 (all m, 12H, NCH2CH3 + CH2CH(P(O)Ph2)–); 4.17 (ddd, 2H, PCH, 2JPH = 13.5, 3JHH = 11.1); 5.45 (dd, 4H, –CH2–Ar–CH2, 2JHH = 14.9, 2JHH = 18.0); 7.21 and 7.44 (both d, 4H + 4H, H(C2) + H(C3) + H(C6) + H(C7) + H(C9) + H(C10) + H(C11) + H(C12) in Ar, 3JHH = 8.4); 7.28 (br. s, 2H, CH in triazole cycle); 7.45–7.55, 7.84–7.90, 8.06–8.12 (all m, 12H + 4H + 4H, C6H5–P). 31P NMR (161.97 MHz, CDCl3): 29.95 ppm. 13C NMR (100.61 MHz, CDCl3, ppm, J/Hz): 12.44 and 13.79 (both s, CH3CH2N), 24.75 (s, CH2CH(P(O)Ph2–), 40.83 and 42.55 (both s, CH3CH2N), 45.79 (d, P–CH, 1JPC = 60.9), 53.23 (br. s, –CH2–Ar–CH2–), 121.95 (s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– in triazole cycle), 127.55 (s, C3, C6, C10, C11 in Ar), 128.48 (s, C2, C7, C9, C12 in Ar), 128.28 and 128.47 (both d, o-C in C6H5, 2JPC = 11.7), 130.73 and 130.86 (both d, ipso-C in C6H5, 1JPC = 98.7), 131.70 and 131.87 (both d, m-C in C6H5, 3JPC = 9.1), 131.94 and 132.02 (both d, p-C in C6H5, 4JPC = 3.0), 133.95 and 140.61 (both s, ipso-C in Ar), 145.05 (d, –C
CH– in triazole cycle), 127.55 (s, C3, C6, C10, C11 in Ar), 128.48 (s, C2, C7, C9, C12 in Ar), 128.28 and 128.47 (both d, o-C in C6H5, 2JPC = 11.7), 130.73 and 130.86 (both d, ipso-C in C6H5, 1JPC = 98.7), 131.70 and 131.87 (both d, m-C in C6H5, 3JPC = 9.1), 131.94 and 132.02 (both d, p-C in C6H5, 4JPC = 3.0), 133.95 and 140.61 (both s, ipso-C in Ar), 145.05 (d, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– in triazole cycle, 3JPC = 15.8), 167.24 (s, C
CH– in triazole cycle, 3JPC = 15.8), 167.24 (s, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). Anal. calcd for C56H60N8O4P2·H2O: C, 68.00; H, 6.32; N, 11.33; P, 6.26. Found: C, 67.84; H, 6.54; N, 11.19; P, 6.21.
O). Anal. calcd for C56H60N8O4P2·H2O: C, 68.00; H, 6.32; N, 11.33; P, 6.26. Found: C, 67.84; H, 6.54; N, 11.19; P, 6.21.Yield: 50%. Mp: 77–87 °C. IR (KBr, cm−1): 3436 (νOH), 2973, 2932, 1631 (νC
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1461 (νAr), 1438, 1214 and 1191 (νP
O), 1461 (νAr), 1438, 1214 and 1191 (νP![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1117, 1099, 1047, 705, 516. 1H NMR (400 MHz, CDCl3, ppm, J/Hz): 0.65 and 0.68 (both t, 6H + 6H, NCH2CH3, 3JHH = 7.2); 2.10 and 2.20 (both s, 6H + 3H, CH3(C2) + CH3(C4) + CH3(C6) in Ar); 2.66–2.75, 2.88–2.98, 3.00–3.15, 3.17–3.27 (all m, 12H, NCH2CH3 + CH2CH(P(O)Ph2)–); 4.09 (ddd, 2H, PCH, 2JPH = 14.0, 3JHH = 11.2); 5.38 (br. s, 4H, –CH2–Ar–CH2–); 6.84 (s, 1H, H(C5) in Ar); 7.43 (s, 2H, CH in triazole cycle); 7.32–7.50, 7.75–7.82, 7.96–8.05 (all m, 12H + 4H + 4H, C6H5–P). 31P NMR (161.97 MHz, CDCl3): 30.09 ppm. 13C NMR (100.61 MHz, CDCl3, ppm, J/Hz): 12.41 and 13.77 (both s, CH3CH2N), 19.71 (s, CH3(C4) + CH3(C6) in Ar), 19.84 (s, CH3(C2) in Ar), 24.71 (s, CH2CH(P(O)Ph2–), 40.79 and 42.50 (both s, CH3CH2N), 45.68 (d, P–CH, 1JPC = 60.5), 48.17 (s, –CH2–Ar–CH2–), 120.61 (s, C5 in Ar), 121.07 (s,
O), 1117, 1099, 1047, 705, 516. 1H NMR (400 MHz, CDCl3, ppm, J/Hz): 0.65 and 0.68 (both t, 6H + 6H, NCH2CH3, 3JHH = 7.2); 2.10 and 2.20 (both s, 6H + 3H, CH3(C2) + CH3(C4) + CH3(C6) in Ar); 2.66–2.75, 2.88–2.98, 3.00–3.15, 3.17–3.27 (all m, 12H, NCH2CH3 + CH2CH(P(O)Ph2)–); 4.09 (ddd, 2H, PCH, 2JPH = 14.0, 3JHH = 11.2); 5.38 (br. s, 4H, –CH2–Ar–CH2–); 6.84 (s, 1H, H(C5) in Ar); 7.43 (s, 2H, CH in triazole cycle); 7.32–7.50, 7.75–7.82, 7.96–8.05 (all m, 12H + 4H + 4H, C6H5–P). 31P NMR (161.97 MHz, CDCl3): 30.09 ppm. 13C NMR (100.61 MHz, CDCl3, ppm, J/Hz): 12.41 and 13.77 (both s, CH3CH2N), 19.71 (s, CH3(C4) + CH3(C6) in Ar), 19.84 (s, CH3(C2) in Ar), 24.71 (s, CH2CH(P(O)Ph2–), 40.79 and 42.50 (both s, CH3CH2N), 45.68 (d, P–CH, 1JPC = 60.5), 48.17 (s, –CH2–Ar–CH2–), 120.61 (s, C5 in Ar), 121.07 (s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– in triazole cycle), 128.19 and 128.40 (both d, o-C in C6H5, 2JPC = 12.1), 129.72 (d, ipso-C in C6H5, 1JPC = 112.2), 131.62 and 131.78 (both d, m-C in C6H5, 3JPC = 9.2), 131.89 and 131.96 (both d, p-C in C6H5, 4JPC = 2.5), 137.71 (s, ipso-C4, C6 in Ar), 137.84 (s, ipso-C2 in Ar), 138.74 (s, ipso-C1, C3 in Ar), 144.32 (d, –C
CH– in triazole cycle), 128.19 and 128.40 (both d, o-C in C6H5, 2JPC = 12.1), 129.72 (d, ipso-C in C6H5, 1JPC = 112.2), 131.62 and 131.78 (both d, m-C in C6H5, 3JPC = 9.2), 131.89 and 131.96 (both d, p-C in C6H5, 4JPC = 2.5), 137.71 (s, ipso-C4, C6 in Ar), 137.84 (s, ipso-C2 in Ar), 138.74 (s, ipso-C1, C3 in Ar), 144.32 (d, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– in triazole cycle, 3JPC = 15.8), 167.18 (s, C
CH– in triazole cycle, 3JPC = 15.8), 167.18 (s, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). Anal. calcd for C53H62N8O4P2·1.5H2O: C, 66.03; H, 6.80; N, 11.62; P, 6.43. Found: C, 66.23; H, 6.75; N, 11.58; P, 6.30.
O). Anal. calcd for C53H62N8O4P2·1.5H2O: C, 66.03; H, 6.80; N, 11.62; P, 6.43. Found: C, 66.23; H, 6.75; N, 11.58; P, 6.30.Yield: 65%. Mp: 129–130 °C. IR (KBr, cm−1): 3436 (νOH), 2971, 2930, 1631 (νC
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1461 (νAr), 1438, 1217 and 1191 (νP
O), 1461 (νAr), 1438, 1217 and 1191 (νP![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1117, 1099, 705, 516. 1H NMR (400 MHz, CDCl3, ppm, J/Hz): 0.77 (br. t, 12H, NCH2CH3, 3JHH = 7.6); 2.20 (s, 12H, CH3(C2) + CH3(C3) + CH3(C5) + CH3(C6) in Ar); 2.76–2.90 and 3.00–3.40 (both m, 12H, NCH2CH3 + CH2CH(P(O)Ph2)–); 4.21 (ddd, 2H, PCH, 2JPH = 14.0, 3JHH = 11.2); 5.57 (s, 4H, –CH2–Ar–CH2–); 6.93 (s, 2H, CH in triazole cycle); 7.45–7.65, 7.82–7.95, 8.06–8.18 (all m, 12H + 4H + 4H, C6H5–P). 31P NMR (161.97 MHz, CDCl3): 30.24 ppm. 13C NMR (100.61 MHz, CDCl3, ppm, J/Hz): 12.44 and 13.81 (both s, CH3CH2N), 16.33 (s, CH3(C2) + CH3(C3) + CH3(C5) + CH3(C6) in Ar), 24.79 (s, CH2CH(P(O)Ph2–), 40.87 and 42.61 (both s, CH3CH2N), 45.73 (d, P–CH, 1JPC = 80.5), 48.90 (s, –CH2–Ar–CH2–), 121.20 (s,
O), 1117, 1099, 705, 516. 1H NMR (400 MHz, CDCl3, ppm, J/Hz): 0.77 (br. t, 12H, NCH2CH3, 3JHH = 7.6); 2.20 (s, 12H, CH3(C2) + CH3(C3) + CH3(C5) + CH3(C6) in Ar); 2.76–2.90 and 3.00–3.40 (both m, 12H, NCH2CH3 + CH2CH(P(O)Ph2)–); 4.21 (ddd, 2H, PCH, 2JPH = 14.0, 3JHH = 11.2); 5.57 (s, 4H, –CH2–Ar–CH2–); 6.93 (s, 2H, CH in triazole cycle); 7.45–7.65, 7.82–7.95, 8.06–8.18 (all m, 12H + 4H + 4H, C6H5–P). 31P NMR (161.97 MHz, CDCl3): 30.24 ppm. 13C NMR (100.61 MHz, CDCl3, ppm, J/Hz): 12.44 and 13.81 (both s, CH3CH2N), 16.33 (s, CH3(C2) + CH3(C3) + CH3(C5) + CH3(C6) in Ar), 24.79 (s, CH2CH(P(O)Ph2–), 40.87 and 42.61 (both s, CH3CH2N), 45.73 (d, P–CH, 1JPC = 80.5), 48.90 (s, –CH2–Ar–CH2–), 121.20 (s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– in triazole cycle), 128.24 and 128.47 (both d, o-C in C6H5, 2JPC = 16.1), 130.77 and 130.69 (both d, ipso-C in C6H5, 1JPC = 132.4), 131.21 (s, ipso-C2, C3, C5, C6 in Ar), 131.75 and 131.80 (both d, m-C in C6H5, 3JPC = 12.0), 132.00 (br. s, p-C in C6H5), 134.72 (s, ipso-C1, C4 in Ar), 144.31 (d, –C
CH– in triazole cycle), 128.24 and 128.47 (both d, o-C in C6H5, 2JPC = 16.1), 130.77 and 130.69 (both d, ipso-C in C6H5, 1JPC = 132.4), 131.21 (s, ipso-C2, C3, C5, C6 in Ar), 131.75 and 131.80 (both d, m-C in C6H5, 3JPC = 12.0), 132.00 (br. s, p-C in C6H5), 134.72 (s, ipso-C1, C4 in Ar), 144.31 (d, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– in triazole cycle, 3JPC = 21.2), 167.25 (s, C
CH– in triazole cycle, 3JPC = 21.2), 167.25 (s, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). Anal. calcd for C54H64N8O4P2·H2O: C, 66.93; H, 6.86; N, 11.56; P, 6.39. Found: C, 66.78; H, 6.81; N, 11.62; P, 6.25.
O). Anal. calcd for C54H64N8O4P2·H2O: C, 66.93; H, 6.86; N, 11.56; P, 6.39. Found: C, 66.78; H, 6.81; N, 11.62; P, 6.25.Extraction studies
In the extraction experiments, 1,2-dichloroethane of chemical grade without any additional purification was used as an organic solvent. Extractant solutions were prepared using precisely weighed samples. Stock aqueous solutions of lanthanides(III), U(VI), Th(IV) and Pd(II) were prepared by dissolving the corresponding nitrates in water followed by the addition of HNO3. The initial concentration of metal ions was 1 × 10−5 M. All lanthanides(III) (except Pm) were present in the initial aqueous phase when simultaneous extraction of Ln(III) was studied. The extraction experiments were performed in tubes equipped with sealing plugs at room temperature and the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio of organic and aqueous phases. The phases were contacted in a rotar mixer at the rate 60 rpm for 1 h, this time being sufficient to reach constant values of the distribution ratio (D).
1 volume ratio of organic and aqueous phases. The phases were contacted in a rotar mixer at the rate 60 rpm for 1 h, this time being sufficient to reach constant values of the distribution ratio (D).
The concentration of Ln(III), U(VI), Th(IV) and Pd(II) in the initial and equilibrium aqueous solutions was determined using inductively coupled plasma mass spectrometry (ICP-MS) on a mass spectrometer X-7 (Thermo Electron, USA) according to the previously described procedure.22 The content of U, Th and lanthanides in the organic phase was determined after back-extraction with 0.1 M solution of 1-hydroxyethane-1,1-diphosphonic acid. The distribution ratio of metal ions was calculated as a ratio of their concentrations in the equilibrium phases. Duplicate experiments showed that the reproducibility of the D measurements was generally within 10%. The concentration of HNO3 in the equilibrium aqueous phase was determined by potentiometric titration with KOH solution.
Results and discussion
Characterization of compounds 1–6
The pure ligands 1–6 demonstrate a singlet at ca. 30 ppm in the 31P NMR spectrum, i.e., the region characteristic for the given phosphorus atom environment. The 1H NMR spectrum corresponds to the depicted structure and is comprises a doublet doublet doublet (ddd) of protons of the PCH group at ca. 4.17 ppm with coupling constants of 11.0 and 14.2 Hz along with the other characteristic signals of the hydrogen atoms located in the typical regions. In the IR spectrum characteristic absorption bands at 1631 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1461 cm−1 (Ar) and 1215 cm−1 (P
O), 1461 cm−1 (Ar) and 1215 cm−1 (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) along with the absorption of OH group appearing as a broad band at 3436 cm−1 (valent oscillations) were observed.
O) along with the absorption of OH group appearing as a broad band at 3436 cm−1 (valent oscillations) were observed.
Extraction of Pd(II) from HNO3 and HCl solutions
To compare the extraction efficiency of compounds 1–8 towards Pd(II), the DPd values were measured at the Pd(II) extraction from 3 M HNO3 containing 0.001 M Pd(II) with 0.001 M solutions of compounds 1–7 and 0.01 M compound 8 in 1,2-dichloroethane. The data in Table 1 show that the extraction efficiency of compounds 1–7 towards Pd(II) is considerably higher than that for compound 8. This suggests the participation of a 1,2,3-triazole fragment of extractants 1–7 molecule in the complexation with Pd2+ ions. Earlier, the formation of Pd–N bonds was reported by Khisamutdinov et al.23 for complexes of Pd(II) with 1-2(2,4-dichlorphenyl)-propyl-1,3-dioxolan-2-yl-methyl-1H-1,2,4-triazol. However, it is difficult to discuss the participation of CMPO moieties in the complex formation with Pd2+ ions in detail because of the lack of information on the real coordination fashion of extracted complexes since no direct structural parameters under the same extraction conditions have been obtained yet.| Extractant | lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPd DPd |
Extractant | lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPd DPd |
|---|---|---|---|
| 1 | 1.48 | 5 | 1.62 |
| 2 | 1.70 | 6 | 1.79 |
| 3 | 1.60 | 7 | 1.33 |
| 4 | 0.92 | 8 | −1.27 |
The data in Table 1 show that the efficiency of Pd(II) extraction with compounds 1–3, 5 and 6 containing two triazole fragments is higher than that for their mono analog 7. Pd(II) forms square-planar complexes, which could make the symmetrical phenyl-bridged bis-CVPO-triazol ligands especially effective for the extraction of Pd(II).
A similar increase in the efficiency of Pd(II) extraction was observed on going from benzoylthiourea to 1-benzoyl-3[6-(3-benzoyl-thioureido)-hexyl]-thiourea.24 However, the extraction efficiency of compound 4 with biphenyl spacer between triazole groups is lower than that for its mono analog 7. This can be due to a larger distance between triazole fragments, as compared with compounds 1–3, 5 and 6.
The effect of HNO3 and HCl concentrations in the equilibrium aqueous phase on the extraction of Pd(II) ions with solutions of compound 6 in 1,2-dichloroethane is shown in Fig. 1.
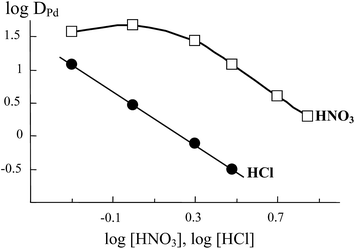 | ||
| Fig. 1 The effect of HNO3 and HCl concentration in the aqueous phase on the extraction of Pd(II) with 2 × 10−5 M solutions of compound 6 in 1,2-dichloroethane. Solid lines are guide for the eyes. | ||
The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPd vs. log[HNO3] curve exhibits a maximum at [HNO3] = 1 M. This can be due to the combined effect of HNO3 salting out and competition for the extractant, as well as to the formation of Pd(II) anionic complexes in the aqueous phase.25 The data in Fig. 1 suggest that compound 6 extracts Pd(II) from HNO3 solutions more effectively than from HCl solutions. This is connected with a high stability of [PdCl4]2− complexes in the aqueous phase.25
DPd vs. log[HNO3] curve exhibits a maximum at [HNO3] = 1 M. This can be due to the combined effect of HNO3 salting out and competition for the extractant, as well as to the formation of Pd(II) anionic complexes in the aqueous phase.25 The data in Fig. 1 suggest that compound 6 extracts Pd(II) from HNO3 solutions more effectively than from HCl solutions. This is connected with a high stability of [PdCl4]2− complexes in the aqueous phase.25
The stoichiometric ratio of palladium(II) to extractant in the extracted complexes was determined by the slope analysis method. The variations in DPd as a function of compounds 6 and 7 concentration in 1,2-dichloroethane for 3 M HNO3 solutions are shown in Fig. 2.
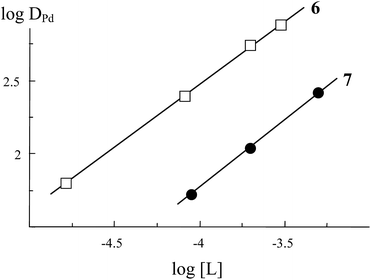 | ||
| Fig. 2 The effect of extractants 6 and 7 concentration in 1,2-dichloroethane on the extraction of Pd(II) from 3 M HNO3 solutions. Solid lines: linear variation with a slope of 0.94 ± 0.06. | ||
The dependence log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPd vs. log([L]init − s[Pd]o), where [L]init is the initial extractant concentration and [Pd]o is the equilibrium concentration of Pd(II) in the organic phase, is linear with a slope of 1. Hence, one molecule of compounds 6 and 7 are involved in the formation of complexes with Pd(II) under the experimental conditions used.
DPd vs. log([L]init − s[Pd]o), where [L]init is the initial extractant concentration and [Pd]o is the equilibrium concentration of Pd(II) in the organic phase, is linear with a slope of 1. Hence, one molecule of compounds 6 and 7 are involved in the formation of complexes with Pd(II) under the experimental conditions used.
Extraction of U(VI), Th(IV) and Ln(III) from nitric acid solutions
The effect of HNO3 concentration in the equilibrium aqueous phase on the extraction of U(VI), Th(IV) and Eu(III) ions with solutions of compound 6 in 1,2-dichloroethane is shown in Fig. 3. An increase in HNO3 concentration is accompanied by the growth of distribution ratios of U(VI) and Th(IV).A similar character of the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D–log[HNO3] dependence was observed for the extraction of U(VI) and Pu(IV) with CMPO extractants in the form of coordination-solvated nitrates.26 The log
D–log[HNO3] dependence was observed for the extraction of U(VI) and Pu(IV) with CMPO extractants in the form of coordination-solvated nitrates.26 The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEu vs. log[HNO3] curve exhibits a maximum in the range of [HNO3] between 2 and 3 M (Fig. 3). These maxima are commonly observed in the Ln(III) extraction with neutral organophosphorus compounds and are explained by the combined effect of HNO3 salting out and competition for extractant, as well as by the change of the activity coefficient values of Ln(III) nitrates depending on [HNO3].27
DEu vs. log[HNO3] curve exhibits a maximum in the range of [HNO3] between 2 and 3 M (Fig. 3). These maxima are commonly observed in the Ln(III) extraction with neutral organophosphorus compounds and are explained by the combined effect of HNO3 salting out and competition for extractant, as well as by the change of the activity coefficient values of Ln(III) nitrates depending on [HNO3].27
The variations in DU and DTh as a function of extractants 1–6 concentration in 1,2-dichloroethane are shown in Fig. 4. The first power dependence of DU and DTh on [L] points out that U(VI) and Th(IV) ions are extracted with compounds 1–6 from nitric acid solutions mostly as monosolvates. In contrast to the above compounds, their mono analogs compounds 7 (ref. 6) and 8 (ref. 26) extract U(VI) and Th(IV) as a mixture of mono- and disolvates under similar conditions.
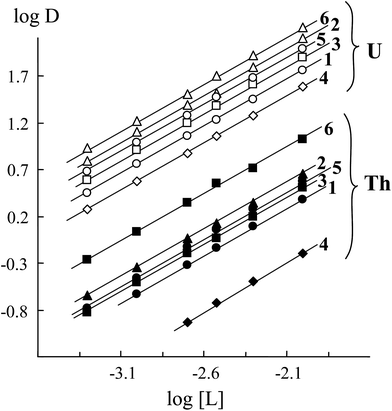 | ||
| Fig. 4 The effect of extractants 1–6 concentration in 1,2-dichloroethane on the extraction of U(VI) and Th(IV) from 3 M HNO3 solutions. Solid lines: linear variation with a slope of 0.98 ± 0.06. | ||
For the lanthanides(III) extraction with compound 6, a non-integer slope of the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DLn vs. log[L] curves is observed (Fig. 5). This can be a result of the formation of a mixture of mono- and disolvates in the organic phase. In this case, the relation between DLn and the extractant concentration in the organic phase can be presented as
DLn vs. log[L] curves is observed (Fig. 5). This can be a result of the formation of a mixture of mono- and disolvates in the organic phase. In this case, the relation between DLn and the extractant concentration in the organic phase can be presented as
| DLn = [NO3−]3(K1[L] + K2[L]2) |
 | ||
| Fig. 5 The effect of extractants 6 concentration in 1,2-dichloroethane on the extraction of Ln(III) from 3 M HNO3 solutions. Solid lines: linear variation with a slope of 1.48 ± 0.12. | ||
Under the same conditions, compounds 1–5 also extract Ln(III) as a mixture mono- and disolvates. In contrast to above compounds, their mono analogues compounds 7 (ref. 6) and 8 (ref. 28) extract Ln(III) as disolvates and a mixture of di- and trisolvates, respectively. The decrease in the solvate numbers in the extracted Ln(III) complexes with compounds 1–6 may be caused by polydentate coordination of the ligands during complexation with Ln(III) ions.
To compare the extraction efficiency of compounds 1–8 towards U(VI), Th(IV) and lanthanide(III) ions in nitric acid systems, the DU, DTh and DEu values were measured at the extraction with 0.01 M solutions of compounds 1–8 in 1,2-dichloroethane from 3 M HNO3 solutions. The data in Table 2 suggest that the introduction of the 1,2,3-triazole fragment into the methylene bridge a CMPO molecule 8 does not essentially change the extraction efficiency of compound 7 towards U(VI) and Eu(III) but drastically decreases the Th(IV) extraction with this compound. Evidently, the extraction of Th(IV) is more sensitive to an increase in the conformational rigidity of an extractant molecule. In contrast to unsubstituted CMPO 8, compounds 1–7 extract U(VI) more effectively than Th(IV). The difference in the extractability of U(VI) and Th(IV) by compounds 8 and 1–7 may be due to the difference in the geometry of extracted complexes.
| Extractant | lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DU DU |
lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DTh DTh |
lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEu DEu |
|---|---|---|---|
| 1 | 1.76 | 0.38 | −1.26 |
| 2 | 2.10 | 0.66 | −0.94 |
| 3 | 1.90 | 0.51 | −1.14 |
| 4 | 1.59 | −0.20 | −1.93 |
| 5 | 1.99 | 0.57 | −1.03 |
| 6 | 2.22 | 1.02 | −0.94 |
| 7 | 1.25 | 0.01 | −1.86 |
| 8 | 1.27 | 3.10 | −1.78 |
Bis-CMPO compounds 1–3, 5 and 6 extract U(VI), Th(IV) and Eu(III) more effectively than their mono-CMPO analog 7. Evidently, both CMPO fragments of an extractant molecule take part in the complexation with metal ions. However, the extraction efficiency of bis-CMPO 4 with biphenyl spacer between triazole fragments does not increase in the case of Eu(III), moreover it becomes lower for Th(IV) as compared with that of compound 7. This can be a result of an increase in the distance between CMPO fragments in the compound 4 molecule and, probably, of a greater conformational rigidity, as compared with compounds 1–3, 5 and 6.
Compound 2 extracts U(VI), Th(IV) and Eu(III) more effectively than its o- and p-isomers – compounds 1 and 3. The same effect was observed on the extraction of Am(III) and Eu(III) with bis(diarylphosphorylmethyl)benzenes from nitric acid solutions, but in a significantly higher degree: on going from ortho- to meta-isomers, DAm and DEu values are three orders of magnitude higher.29,30
The effect of the structure of compounds 2, 7 and 8 on the efficiency of lanthanides(III) ions extraction was examined by performing simultaneous extraction of Ln(III) ions from 3 M HNO3 solutions with these compounds in 1,2-dichloroethane (Fig. 6).
 | ||
| Fig. 6 The extraction of lanthanides(III) from 3 M HNO3 solutions with 0.05 M solutions of compounds 7–9 and 2 in 1,2-dichloroethane. The data for 9 from ref. 31. Solid lines are guide for the eyes. | ||
The figure also presents our previous data31 on the Ln(III) extraction with 1,7-bis(dibutylcarbamoyl)1,7bis(diphenylphosphinyl)-heptane 9, whose molecule contains two Ph2P(O)CHC(O)NBu2 coordinating groups linked by a pentamethylene spacer through methylene groups. At this HNO3 concentration in the equilibrium aqueous phase, the extraction of Ln(III) decreases from La(III) to Lu(III). In general, such lanthanide pattern is typical for the ligands of the CMPO type32,33 as well as for some other neutral bidentate ligands, such as tetraarylmethylenediphosphine dioxides34,35 and alkylsubstituted malonamides36 in nitric acid systems. These trends were explained by the rise in the hydration energies of Ln(III) ions as their ionic radii decrease.32
The data in Fig. 6 show that DLn values for compound 7 are lower than those for compound 8. That is, the replacement of one hydrogen atom in the methylene bridge of CMPO molecule by a triazole fragment decreases the extraction efficiency of compound 7 towards Ln(III). The same effect was observed on the extraction of Am(III) and Eu(III) with tetraphenyl-methylenediphosphine dioxide37 and CMPO38 with alkyl or other substituents in the methylene bridge between coordinating groups. This was assigned to a limited conformational mobility of the spacer between P(O) and C(O) groups of the extractant molecule and an associated inhibition of the formation of a six-membered chelate complex with the extracted ion.38 This can probably explain the lower extraction efficiency of bis-CMPO 9 towards Sm–Lu than that of its monoanalog 8.
The data in Fig. 6 suggest that, under comparable conditions, the extraction efficiency of bis-CMPO 2 is significantly higher than that of its monoanalog 7. The DLn values for compound 2 are higher than those for bis-CMPO 9 with pentamethylene spacer between CMPO moieties. However, it is difficult to discuss the participation of 1,2,3-triazole groups in the complexation with Ln(III) ions in detail because of the lack of information on the real coordination fashion of extracted complexes since no direct structural parameters under the same extraction conditions have been obtained yet.
Conclusions
Novel polyfunctional neutral organophosphorus compounds – Ar-CMPO were synthesized and studied as extractants for Pd(II), Ln(III), U(VI) and Th(IV) from HNO3 solutions. The influence of aqueous and organic phases on the extraction efficiency was elucidated and stoichiometry of the complexes extracted was determined. The data obtained showed that one of the ways to modify CMPO aimed at the improvement of their extraction ability towards Pd(II) is to introduce 1,2,3-triazole fragment into the methylene bridge of the CMPO molecule. This modification of an extractant molecule does not essentially change the extraction efficiency towards U(VI) and Ln(III) but drastically decreases the Th(IV) extraction and change the order in the extractability of U(VI) and Th(IV) from HNO3 solutions. An increase in the number of CMPO- and triazole fragments in extractants molecule leads to an increase of their extraction efficiency towards Pd(II), Ln(III), U(VI) in nitric acid systems.Acknowledgements
The work was carried out with financial support from the Ministry of Education and Science of the Russian Federation in the framework of Increase Competitiveness Program of NUST « MISiS» (no. K1-2014-026).Notes and references
- Z. Kolaric and E. V. Renard, Platinum Met. Rev., 2003, 47, 74 Search PubMed.
- E. P. Horwitz, and W. W. Schulz, Metal Ion Separation and Preconcentration: Progress and Opportunities, ed. A. H. Bond, M. L. Dietz and R. D. Rogers, ACS Symposium Series 712, American Chemical Society, Washington, D.C., 1999, p. 20 Search PubMed.
- M. K. Chmutova, M. N. Litvina, G. A. Pribilova, L. A. Ivanova, I. B. Smirnov, A. Yu. Shadrin and B. F. Myasoedov, Radiokhimiya, 1999, 41, 331 Search PubMed.
- Z.-X. Zhu, Y. Sasaki, H. Suzuki, S. Suzuki and T. Kimura, Anal. Chim. Acta, 2004, 527, 163 CrossRef CAS PubMed.
- Y. Sasaki, M. Ozawa, T. Kimura and K. Ohashi, Solvent Extr. Ion Exch., 2009, 27, 378 CrossRef CAS.
- A. N. Turanov, V. K. Karandashev, O. I. Artyushin, E. V. Sharova, G. K. Genkina and A. N. Yarkevich, Solvent Extr. Ion Exch., 2014, 32, 669 CrossRef CAS.
- A. N. Turanov, V. K. Karandashev, E. V. Sharova, O. I. Artyushin and I. L. Odinets, Solvent Extr. Ion Exch., 2010, 28, 579 CrossRef CAS.
- A. N. Turanov, V. K. Karandashev, E. V. Sharova, O. I. Artyushin and I. L. Odinets, Solvent Extr. Ion Exch., 2012, 30, 604 CrossRef CAS.
- A. Arduini, V. Bochmer, L. Delmau, J. F. Desreux, J.-F. Dozol, A. G. Carrera, B. Lambert, C. Musigmann, A. Pochini, A. Shivanyuk and F. Ugozzoli, Chem.–Eur. J., 2000, 6, 2135 CrossRef CAS.
- L. Atamas, O. Klimchuk, V. Rudzevich, V. Pirozhenko, V. Kalchenko, I. Smirnov, V. Babain, T. Efremova, A. Varnek, G. Wipff, F. Arnaud-New, M. Roch, M. Saadioui and V. Bohmer, J. Supramol. Chem., 2002, 2, 421 CrossRef CAS.
- L. H. Delmau, N. Simon, M.-J. Schwing-Weill, F. Arnaud-New, J.-F. Dozol, S. Eymard, B. Tournois, C. Gruttner, C. Musigmann, A. Tunayar and V. Bohmer, Sep. Sci. Technol., 1999, 43, 863 CrossRef.
- H. Boerrigter, W. Verboom and D. N. Reinhoudt, J. Org. Chem., 1997, 62, 7148 CrossRef CAS PubMed.
- M. M. Reinoso-Garcia, W. Verboom, D. N. Reinhoudt, F. Brisach, F. Arnaud-New and K. Liger, Solvent Extr. Ion Exch., 2005, 23, 425 CrossRef CAS PubMed.
- P. Amrhein, P. L. Wash, A. Shivanyuk and J. Rebek, Org. Lett., 2002, 4, 319 CrossRef CAS PubMed.
- P. Amerhein, A. Shivanyuk, D. W. Jonson and J. Rebek, J. Am. Chem. Soc., 2002, 124, 10349 CrossRef PubMed.
- A. N. Turanov, V. K. Karandashev, A. V. Kharitonov, A. N. Lezhnev, Z. V. Safronova, A. N. Yarkevich and E. N. Tsvetkov, Russ. J. Gen. Chem., 1999, 69, 1068 CAS.
- Z.-F. Guo, H. Yan and Z.-F. Li, Org. Biomol. Chem., 2011, 9, 6788 CAS.
- L. K. Haridas, Y. K. Sharma and S. Upreti, Org. Lett., 2008, 10, 1645 CrossRef PubMed.
- M. Pascu, A. Ruggi, R. Scopelliti and K. Severin, Chem. Commun., 2013, 49(1), 45 RSC.
- H. R. Seong, D.-S. Kim, S.-G. Kim, H.-J. Choi and K. H. Ahn, Tetrahedron Lett., 2004, 45(4), 723 CrossRef CAS PubMed.
- A. J. Papa, J. Org. Chem., 1966, 31, 1426 CrossRef CAS.
- A. N. Turanov, V. K. Karandashev and V. E. Baulin, Solvent Extr. Ion Exch., 1996, 14, 227 CrossRef CAS.
- R. A. Khisamutdinov, Yu. I. Murinov and O. V. Shitikova, Zh. Neorg. Khim., 2007, 52, 1041 CAS.
- A. N. Turanov, V. K. Karandashev and A. N. Proshin, Solvent Extr. Ion Exch., 2008, 26, 360 CrossRef CAS.
- J. C. Bailer, H. J. Emeleus, S. R. Nyholm and A. F. Trotman-Dickenson, Comprehensive Inorganic Chemistry, Pergamon Press Ltd., Oxford, England, 1st edn, 1973, vol. 3 Search PubMed.
- M. N. Litvina, M. K. Chmutova, G. A. Pribilova, N. P. Nesterova and B. F. Myasoedov, Radiokhimiya, 1998, 40, 550 Search PubMed.
- A. M. Rozen and B. V. Krupnov, Russ. Chem. Rev., 1996, 65, 73 Search PubMed.
- A. N. Turanov, V. K. Karandashev, E. V. Sharova, O. I. Artyushin and I. L. Odinets, Tsvetn. Met., 2012, 3, 51 Search PubMed.
- M. K. Chmutova, G. V. Bodrin, M. N. Litvina, A. G. Matveeva, E. I. Matrosov, Yu. M. Polikarpov, P. L. Khiznyak, B. F. Myasoedov and M. I. Kabachnik, Radiokhimiya, 1989, 31, 83 CAS.
- B. F. Myasoedov, G. V. Bodrin, M. K. Chmutova, N. E. Kochetkova, T. Ya. Medved, Yu. M. Polikarpov and M. I. Kabachnik, Solvent Extr. Ion Exch., 1983, 4, 689 CrossRef.
- A. N. Turanov, V. K. Karandashev and A. N. Yarkevich, Radiochemistry, 2012, 54, 477 CrossRef CAS.
- E. P. Horwitz, K. A. Martin, H. Diamond and L. Kaplan, Solvent Extr. Ion Exch., 1986, 4, 449 CrossRef CAS.
- M. N. Litvina, M. K. Chmutova, B. F. Myasoedov and M. I. Kabachnik, Radiokhimiya, 1996, 38, 525 Search PubMed.
- T. Yaita and S. Tachimori, Radiochim. Acta, 1996, 73, 27 CAS.
- A. N. Turanov, V. K. Karandashev and N. A. Bondarenko, Radiochemistry, 2007, 49, 55 CrossRef CAS.
- H. Narita, T. Yaita, K. Tamura and S. Tachimori, J. Radioanal. Nucl. Chem., 1999, 239, 381 CrossRef CAS.
- N. E. Kochetkova, O. E. Koiro, N. P. Nesterova, T. Ya. Medved, M. K. Chmutova, B. F. Myasoedov and M. I. Kabachnik, Radiokhimiya, 1986, 28, 338 CAS.
- B. F. Myasoedov, M. K. Chmutova, N. E. Kochetkova, O. E. Koiro and G. A. Pribilova, Solvent Extr. Ion Exch., 1986, 4, 61 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |









