The effects of long chain branching of polypropylene and chain extension of poly(ethylene terephthalate) on the thermal behavior, rheology and morphology of their blends
Yousef Jahani*a,
Milad Ghetmirib and
Mohammad Rahim Vaseghib
aFaculty of Polymer Processing, Iran Polymer and Petrochemical Institute, Tehran, Iran. E-mail: y.jahani@ippi.ac.ir
bDepartment of Polymer Engineering, Shiraz Branch, Islamic Azad University, Shiraz, Iran
First published on 19th February 2015
Abstract
In this work the blends of long chain branched polypropylene (BPP) and chain extended/branched PET (MPET) were prepared and the melt and crystallization characteristics, rheological properties in shear and elongational mode for BPP, MPET and their blends in correlation with their morphology were studied with various amounts of PP grafted maleic anhydride as compatibilizer. BPP is prepared by using dicumyl peroxide and trifunctional monomer trimethylolpropane trimethacrylate (TMPTMA) and MPET were prepared by using tetrafunctional modifier pyromellitic dianhydride (PMDA) and pentaerythritol (PENTA). The rheological study showed the increase in melt shear and elongational strengths of MPET after the chain extension process and increase in shear thinning and strain hardening behavior of modified samples. The zero shear viscosity and shear thinning index of PET increased from 210 Pa s and n′ = 0.15 to 315 Pa s and n′ = 0.22 after modification and for PP they decreased from 4940 Pa s and n′ = 0.54 to 1170 Pa s and n′ = 0.45. These results prove that increase in molecular weight and widening of molecular weight distribution are the major consequences of PET modification and chain scission; narrower MWD and long chain branching are the main phenomena of branching of PP. A slight nucleation effect of MPET on BPP was observed and its crystallization temperature (Tc) increased from 119.7 to 121 °C. By increasing compatibility and decreasing heterogeneity, the Tc of BPP slightly decreased and due to the reaction of MPET and maleic anhydride functional groups, the chain mobility was restricted and crystallization and fusion enthalpies decreased.
Introduction
Polypropylene (PP) and polyethylene terephthalate (PET) are the two most widely used thermoplastics in various packaging applications. Due to their linear structures, PP and PET have low melt strength and exhibit no strain hardening behaviour in the melt state and cannot easily be used in processes where elongational flows are dominant, such as foaming and thermoforming. The improvement of melt strength in linear polymers could be obtained by increasing molecular weight (MW), broadening molecular weight distribution (MWD) and introducing long chain branches to the backbone.1,2PP and PET are semicrystalline plastics with low melt elongational strength due to their linear molecular structure. A number of studies have been conducted to enhance the melt strength of polypropylene in the melt state,3,4 or in the solid state by using electron beam or gamma ray irradiation.5,6 These methods result in the grafting of long chain branches on the PP backbone. Chain scission and crosslinking are two main side reactions which may take place simultaneously with branching in PP and can be controlled by chemical structure of low molecular weight additives in melt state branching as well as the irradiation and post-treatment conditions in solid state branching process.7 The efficiency of long chain branching of polypropylene increase by using polyfunctional monomer8 and the materials known as iniferters such as thiuram disulfides9 and dithiocarbamates,10 which are free radical initiators, transfer agents and terminator that are generally used to decrease the free radical residues and suppress the degradation reaction.
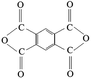
Reactive melt modification of PET with di- and multifunctional monomers is a well-known method to enhance molecular weight and to create long-chain branched structures in PET which leads to enhanced elongational properties and strain hardening behaviour.11,12 Pyromellitic dianhydride (PMDA) is one of the most attractive and economical functional monomer to modify PET. It is thermally stable, produces no side products and may also scavenge the water of alcoholises which may leads to further hydrolysis reactions and decreases in molecular weight.13
Depending on the operation time and temperature, concentration and structure of multifunctional monomers chain extension, branching and cross-linking reactions may occur.14 It has been found that using pentaerythritol (PENTA) together with PMDA leads to higher melt strength and viscosity than sole PMDA,13 and lower crystallinity were obtained.15
The recent study on the PET modified with high PMDA contents by size exclusion chromatography and transient elongational rheometry revealed that the modified PET has a treelike branch-on-branch architecture, which is well-known from low-density polyethylene melt.12
The enhanced melt elongational strength of modified PET improve its thermoformability and foamability. It is found a considerable improve in foamability for PETs modified with up to 0.8 wt% PMDA using supercritical carbon dioxide as blowing agent with broad foaming temperature windows and with the expansion ratio between 10 and 50 times.16
The blends of polyolefins and PET are of clear technological interest. Due to low compatibility of polyolefins with other polar polymers it is proven to use block or graft polymers as compatibilizer to enhance the interfacial adhesion and improve the properties.17 The graft copolymers suggest advantages to enhance the interfacial adhesion by localizing at the interface and reducing interfacial tension in heterogeneous polymer blends.18
It has been observed that the type and structure of different compatibilizer have significant influence on the compatibility of PP/PET blends.19,20
The reactive melt blending of linear PP and PET with various types and amounts of compatibilizer was the subject of many research works.21,22 The influence of viscosity of phases, reactive groups, molecular weight on the rheology and morphology of PP/PET blends was extensively studied in recent years.20 However, no report is found relating to the blends of long chain branched PP with chain extended/branched PET so far.
In this research the long chain branched PP were prepared in melt state and PET were chain extended/branched by PMDA and PENTA. The long chain branched PP and modified PET (MPET) were mixed in melt state by passing through twin screw extruder with various amounts of polypropylene grafted maleic anhydride as compatibilizer. To evaluate the branching and modification process and to study the effect of compatibilizer the shear and uniaxial elongational rheology morphology and thermal behaviour of samples were investigated.
Experimental
Materials
The polypropylene used in this study is an isotactic homopolymer PP (Moplen HP500H) with melt flow rate (MFR) of 2.15 g/10 min supplied by Arak petrochemical company, Iran. Bottle grade PET with an intrinsic viscosity of 0.78 (dl g−1) supplied by Tondgooyan petrochemical company, Iran. PMDA Sigma Aldrich as a chain extender and PENTA (Sigma Aldrich) were used to promote branching reactions in PET. Dicumyl peroxide (DCP 99%, Arkema) as an initiator, Trimethylolpropane trimethacrylate multifunctional monomer (TMPTMA, Sigma Aldrich) and tetramethylthiuram disulfide as an iniferter (TMTD, Sigma Aldrich) were used to increase the efficiency of long chain branching process of PP. Table 1 shows the chemical structure of modifiers used in this work. All the modifiers were used as received. The Fusabond P MD353D (DuPont), a polypropylene grafted with 1.4% of maleic anhydride is used as compatibilizer.Modification of PET (MPET)
The stoichiometric amount of PMDA (0.35 wt%) and 0.07 wt% of PENTA were physical mixed with dried PET granules prior to being fed to the extruder.In order to avoid hydrolysis of PET during mixing, PET granules were dried under vacuum at 70 °C for 6 hours before melt modification process. The reactive modification of PET was carried out in Brabender lab co-rotating twin screw extruder (D = 25 mm, L/D = 40) with a temperature settings of 270–290–280–275–270 °C along the extruder barrel, from hopper to die.
Branching of PP (BPP)
For long chain branching of PP, it was tumble-mixed with DCP initiator, TMPTMA multifunctional monomer and TMTD iniferter with concentration of 0.3, 1.5 and 0.07 phr (parts per hundred resin) respectively.A calibration curve is built to correlate the MFI of PP with various amounts of DCP. The DCP concentration is selected according to the calibration curve for targeted MFI = 7 ± 0.5 g/10 min for PP which is normally convenient for extrusion and injection moulding process.
The amount of TMPTMA is selected by measuring the gel content of samples. In this regard, a number of samples were prepared with fixed DCP concentration of 0.3 phr and various concentration of TMPTMA and the gel content of samples were measured. It is observed no gel up to 1.5 phr of TMPTMA. By increasing the TMPTMA content higher than 1.5 phr, the gel starts to form and increased by increasing the multifunctional monomer concentration. The long chain branching of PP was carried out in twin screw extruder with temperature settings of 170–190–200–210–210 °C.
Blending of MPET/BPP
The MPET/BPP blends were prepared according to recipes-Table 2. The dried MPET granules were tumble mixed with granules of BPP and polypropylene grafted polypropylene (PP-g-MA) as compatibilizer and melt blended in twin-screw extruder with temperature settings of 270–290–280–270–260 °C and 100 rpm screw speed. The extrudate were water cooled and pelletized.| Samples | MPET (wt%) | BPP (wt%) | Compatibilizer (wt%) |
|---|---|---|---|
| B1 | 30 | 70 | 0 |
| B2 | 27.5 | 67.5 | 5 |
| B3 | 25 | 65 | 10 |
Intrinsic viscosity
The intrinsic viscosity (IV) of samples dissolved in 50/50 by weight of phenol/1,2-dichlorobenzene mixture is calculated according to ISO 1228 standard by using Solomon–Ciuta equation of a single point measurement. The viscosity molecular weight was determined from Mark–Houwink relation, with the constants α and K equal to 0.68 and 4.69 × 10−2, respectively.15Gel content
The state of cross-linking reaction was evaluated by measuring the un-soluble fraction. The gel content of PP after long chain branching process were determined according to ASTM D2765 by extracting out of 0.3 g of material packed in steel net of 60 meshes in boiling xylene for 24 h. The gel content of MPET was carried out in the mixture of phenol/1,2-dichlorobenze (50/50 by weight) for 2 hours. The percentage of gel was calculated by measuring the mass change after extraction.Thermal analysis
The melting and crystallization behaviour of samples are evaluated by using Netzsch 200F3 differential scanning calorimeter (DSC) with about 10 mg of samples under nitrogen atmosphere. The samples were first heated to 280 °C at 40 °C min−1 heating rate to remove thermal history and eliminate sample shape abnormalities. After 5 minutes holding at this temperature, they cooled down to 25 °C at a rate of 5 °C min−1 to detect the crystallization behaviour such as crystallization temperature (Tc) and the heat of crystallization (ΔHc). In the second heating run they heated up again to 280 °C with the same rate of 5 °C min−1 to detect the melting characteristic, melting point (Tm) and the enthalpy of fusion (ΔHm). The degree of crystallinity χc of samples was calculated with the eqn (1).
 | (1) |
Shear rheology
The small amplitude oscillatory shear (SAOS) test was conducted with a Physica Anton Paar, MCR-300, Germany, on the 25 mm diameter samples with 1 mm gap size. The circular samples were obtained by stamp die cutting of 1 mm thickness compression moulded sheet. The tests were performed in the frequency range 0.01–600 rad s−1 at a fixed strain of 1%, obtained from strain amplitude sweep test at the frequency of 10 rad s−1, to ensure that measurements were carried out within the linear viscoelastic range of deformation. All measurements were carried out at 265 °C under nitrogen atmosphere. Thermal stability of the samples was checked by time sweep test at a constant angular frequency of 1 s−1, strain amplitude of 1% to ensure the consistency of the molecular structure during the time of experiments.Elongational rheology
The transient extensional viscosity was measured in simple extension mode using SER Universal Testing Platform from Xpansion Instruments that was used with MCR301 rheometer and CTD450 convection oven. The measurements were carried out on 3.3 × 19.4 × 1 mm samples at constant strain rates of 0.01 to 1 s−1 and temperature of 180 °C for PP and 270 °C for PET. The test chamber was purged by dry nitrogen to suppress oxidative degradation of the samples.Scanning electron microscopy
The scanning electron microscopy (SEM) experiments were performed in a VEGAII XMU (TESCAN, Czech) at 20 kV to examine the shape and the size of the dispersed phase. The cryo-fractured surface of samples were etched in 50/50 wt% of phenol/1,2-dichlorobenzene solvents and sputter coated by a thin layer of gold. Number average (Rn), volume average (Rv) and polydispersity of particle radius (d) were measured by digital image analysis.7,28Results and discussion
Shear rheology of BPP and MPET
The complex viscosity of PET and PP before and after modification at various angular frequencies in linear viscoelastic range of deformation are shown in Fig. 1.The complex viscosity at low shear rates (η0) showing a Newtonian plateau is a measure of molecular weight of polymers. The zero shear viscosity of PET increase from 240 to 338 Pa s after modification, which is due to chain extension and branching reactions during the extrusion process. PENTA impose chain scission to PET by alcoholises,13 meanwhile PMDA promote chain extension/branching reactions by interacting to end groups of PET or end groups made by chain scission reaction.15,19 Forsythe et al. showed that modification of PET with PMDA and PENTA results in more viscosity, melt strength and higher storage modulus compared with modified PET with sole PMDA. These effects were attributed to hyperbranched structure formed during reactive modification process.13
The intrinsic viscosity of PET before and after modification is measured. It is observed that the intrinsic viscosity of PET increased from 0.78 dl g−1 to 0.82 dl g−1 and the viscosity average molecular weight (Mv) of PET was increased from 6250 to 6620 after modification.
The complex viscosity of PP before and after branching process is shown in Fig. 1-b. As it is seen, the complex viscosity of BPP shifted to lower values in all range of angular frequencies. PP chains are prone to β-scission in the presence of peroxides. It has been shown that the zero shear viscosity decrease for the polymers with high degree of branching and tree-like structure as for low density polyethylene LDPE in comparison with linear PE with similar molecular weight.2 The decrease in shear viscosity during branching of PP can be attributed to degradation reaction simultaneously taking place in branching process. The decrease in zero shear viscosity by increasing branching number is also observed in the ref. 23.
The yield behaviour before Newtonian plateau is seen in a small extent in the η*–ω graph of branched PP in Fig. 1-b which can be due to molecular entanglement of long chain branched parts of macromolecules.
The Newtonian plateau in the η*–ω graph of branched PP reveals the presence of linear PP together with branched ones after branching process.
The shear thinning index of samples were determined by the slope of η*–ω curves at high shear rate region-Table 3. The increase in shear thinning index of PET from 0.15 to 0.22 after modification demonstrate the increased sensitivity of complex viscosity to frequency24 while, the shear thinning index of modified PP (0.45) is lower than linear one (0.54). The molecular weight distribution of PET widens after chain extension process and narrower distribution obtain by branching of PP in solid or melt state.
| Samples | n′ | η0 (Pa s) | λ (s) | ωc (s−1) | Gc (Pa) |
|---|---|---|---|---|---|
| PET | 0.15 | 210 | 0.1 | 600 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
| MPET | 0.22 | 315 | 0.9 | 500 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
| PP | 0.54 | 4940 | 0.8 | 59.2 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
| BPP | 0.45 | 1170 | 0.2 | 240 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
The zero shear viscosity and shear thinning index of PET increased from 210 Pa s and n′ = 0.15 to 315 Pa s and n′ = 0.22 after modification and for PP they are decreased from 4940 Pa s and n′ = 0.54 to 1170 Pa s and n′ = 0.45. These results approve that increase in molecular weight and widening of molecular weight distribution is the major consequence of PET modification and chain scission, narrower MWD and long chain branching are the main phenomenon in branching of PP.
The characteristics of cross-over point of storage (G′) and loss modulus (G′′) in terminal region can also be used to discuss on MW and MWD. Crossing of loss modulus over storage modulus and their intersection is a point that melt elasticity is equal to loss behaviour and the melt shows an elastic dominant behaviour at higher frequencies. The cross-over frequency (ωc) is inversely correlated to longest relaxation time and also molecular weight. Cross-over modulus Gc is a measure of melt elasticity, which decrease by widening the molecular weight distribution.
The cross-over frequency and cross-over modulus of PET and PP before and after modification are listed in Table 3. The cross-over frequency of PET is shifted to lower values from 590 to 505 s−1 after modification which is due to increase in molecular weight after chain extension process. On the other hand, cross-over modulus of modified PET is decreased from 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 680 to 26
680 to 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 410 Pa which approve its broader molecular weight distribution. Chain extension and branching are two major reactions in the modification of PET by PMDA and PENTA that increase the molecular weight distribution. In the branching process of PP, the peroxide induced chain scission and grafting are two main reactions which leads to loss in molecular weight and narrower molecular weight distribution.25 In this regards, the ωc of PP increased from 59 to 240 s−1 and the Gc showed an small increase from 27
410 Pa which approve its broader molecular weight distribution. Chain extension and branching are two major reactions in the modification of PET by PMDA and PENTA that increase the molecular weight distribution. In the branching process of PP, the peroxide induced chain scission and grafting are two main reactions which leads to loss in molecular weight and narrower molecular weight distribution.25 In this regards, the ωc of PP increased from 59 to 240 s−1 and the Gc showed an small increase from 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 to 30
350 to 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 Pa after branching process. Auhl and co-workers have reported the same results in branching of isotactic PP with electron beam irradiation and an slight decrease in polydispersity has been obtained (narrower molecular weight distribution) from 4.2 to 3.6 depending on does rate.26
200 Pa after branching process. Auhl and co-workers have reported the same results in branching of isotactic PP with electron beam irradiation and an slight decrease in polydispersity has been obtained (narrower molecular weight distribution) from 4.2 to 3.6 depending on does rate.26
Rheology of the blends
The complex viscosity, storage and loss modulus of the blends at various angular frequencies is presented in Fig. 2.The complex viscosity of the blends are lower than the two neat components almost in the whole frequency range. This reveals that blends exhibit negative deviation behaviour (NDB) from log additivity rule which is due to immiscibility of PET and PP. Referring to the viscosity of samples it is observed that the plasticization effect of PP-g-MA on the flow behaviour is more significant than its compatibilization role. This could be due to the diminution in the functional group of PET after modification that limits the reaction with MA groups of PP-g-MA and also because of lower viscosity of compatibilizer than BPP and MPET.27 It has been shown that the addition of PP-g-MA more than 2.5 wt% resulted in attenuating the rheological property to un-compatibilized blend.20 As is seen the addition of compatibilizer is intensified the yield phenomenon as usually is seen in emulsion systems. Pracella et al. have used non-functionalized and functionalized copolymers as compatibilizer for PET/PP blends and showed that non-functionalized copolymers had no significant effect on viscosity while functionalized one increased the viscosity at low frequencies.19 It seems that there is a competition between compatibilization role and plasticizing effect of compatibilizer in the blends which affect the blend's interfacial interaction and morphology. The SEM micrographs of the blends with different amount of compatibilizer are presented in Fig. 3 and the corresponding data of droplet size and size distribution are presented in Table 4.
 | ||
| Fig. 3 SEM micrographs of none-compatibilized and compatibilized BPP/MPET (a) B1, (b) B2, and (c) B3. | ||
| Sample | Rn (μm) | Rv (μm) | d (Rv/Rn) |
|---|---|---|---|
| B1 | 1.37 | 2.84 | 2.07 |
| B2 | 0.74 | 1.08 | 1.44 |
| B3 | 0.67 | 1.38 | 2.06 |
The theoretical emulsion models can be used to investigate the extent of interfacial interaction of polymers in the blend by using rheological data. The Palierne model usually uses for immiscible polymer blends containing moderate concentrations (negligible steric interactions) of none-coalescenced spherical droplets with narrow size distribution (Rv/Rn < 2).28
Bousmina extended Kerner's model for viscoelastic emulsion systems and assumed that the polymers are immiscible, the size of droplets is small with no slip at their surface and density force is negligible.29
The experimental data and theoretical prediction of Palierne and Bousmina models for the blends are shown in Fig. 4.
 | ||
| Fig. 4 Comparison of the complex modulus data of blends with Palierne (—) and Bousmina (---) models. | ||
It is seen that both models are failed to predict well the rheological behaviour and overestimated in the whole frequency range. This can be due to the high concentration of dispersed phase and steric interactions of particles. The failure of Palierne model due to the high concentration of dispersed phase for PP/PET blends has been seen by Shi et al.30
Extensional viscosity
The elongation viscosity measurements help to clarify the structural changes during samples modification. The melt elongation strength of a polymer strongly depends on polymers molecular architecture such as molecular weight and also the number, length and distribution of branches along backbone.31 Strain hardening behaviour and a sharp increase in time scale elongational viscosity at various Hencky strain rate usually observe in the polymer with branched structure. As is seen in Fig. 5-a, PP showed distinct strain hardening behaviour after branching which is because of more entanglement in polymer chains. The elongational viscosity of PET before and after modification is shown in Fig. 5-b. The higher extensional viscosity of PET after modification is due to higher molecular weight of MPET. The strain hardening behaviour is detected also for MPET sample which is more evident at high Hencky strain rates. This observation can be attributed to the branching of PET during the modification process. The strain hardening behaviour is a key characteristic in the processes such as foaming and thermoforming.32 The difference in the dependency of strain hardening to strain rate in MPET and branched PP could be due to difference in the type and amount of branched structure formed in the modified samples. Gabriel and Munstedt2 and Malmberg et al.33 have attributed this phenomena to the formation of a few long chain branches with rather high arm mass which typically is seen in metallocene-catalyzed polyethylene.Thermal behaviour
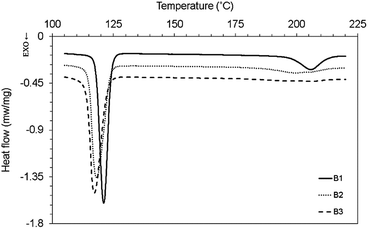
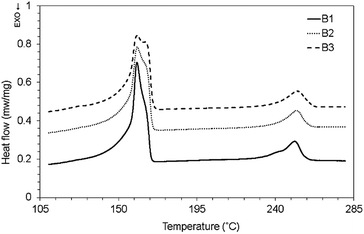
The effect of long chain branching and chain extension process on the melting and crystallization characteristics of PP and PET are shown in Fig. 6-a and b respectively. As is seen, no significant change is observed in the melting point of PP after branching process. It means the segments of macromolecules in the branches were crystallized similar to the macromolecules of the backbone.The melting point of MPET is lower than its linear counterpart with a broader endothermic peak (Fig. 6-b), which owes to the formation of different type of crystals with thinner layer results from defects and irregularities.34 These observations can be due to the different chain structure formed in the chain extension process by different types and amount of chain extenders. The structural changes created by modification process, lead to different types and amount of crystals which affect the melting point and enthalpy of fusion. The shoulder in melting curve of linear PET as a sign of imperfect crystal structures,15 is diminished after modification.
The crystallization temperature of PP increased from 113 °C to 120 °C. The half width of crystallization curve (width of crystallization peak at half height) showed a slight decrease from 6.7 °C to 6.3 °C after branching process which explain nearly similar crystallization rate. The branched points in modified PET structure as nucleation sites promote crystallization initiation rate.
The fusion and crystallization characteristics of samples are listed in Table 5. The effect of reactive modification on the crystallization behaviour of PET is more significant than PP. The crystallization temperature of PET increased from 191.7 °C to about 217 °C and the half width of crystallization curve decreased from 20 °C to 7 °C after modification. It is believed that the branched structure formed during reactive modification process act as heterogeneous nucleating sites with lower free energy35 and affect crystallization kinetics.
| Sample | PET | MPET | PP | BPP | B1 | B2 | B3 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Property | BPP | MPET | BPP | MPET | BPP | MPET | ||||
| Tc (°C) | 191.7 | 216.7 | 112.9 | 119.7 | 121 | 205.2 | 118 | 205.5 | 117.2 | 205.5 |
| ΔHc (J g−1) | 30.2 | 53 | 102.1 | 101.5 | 70.5 | 14.9 | 74.9 | 11.2 | 74.4 | 10.8 |
| Tm (°C) | 252 | 256 | 166.7 | 165.6 | 160.6 | 251 | 160.9 | 252 | 160.7 | 252 |
| ΔHm (J g−1) | 40.1 | 54.8 | 103 | 101.9 | 71 | 15.3 | 75.7 | 13 | 75.4 | 11.2 |
| χc (%) | 33.4 | 45.7 | 49.3 | 48.7 | 48.5 | 42.5 | 51.47 | 36.1 | 51.53 | 31.1 |
PET is a crystalline material with slow crystallization rate at high temperature. Therefore it cannot be used in the injection moulding process without heating the mould by hot oil and considering enough cycle time for proper crystallization. Many attempts have been made to accelerate crystallization of PET by using various types of organic nucleating agent such as sulphonate salt of aromatic dicarboxylic acid,36 and inorganic nucleating agents such as talc, mica.37 The usage of these two types of nucleating agents may face with restriction due to the risk of losing the function of organic ones and aggregation of inorganic types at high processing temperature of PET (about 290 °C). In an attempt to increase the crystallisation rate of PET, the inorganic/organic hybrid oligomer reactive nucleating agents were proposed. This nucleating agent can be dispersed uniformly in PET during in situ polymerization without further process or by blending in an extruder.38 It is observed here a significant improve in crystallization rate, crystallization temperature and also a considerable increase in fusion and crystallization enthalpy of PET after chain extension process. Kiliaris et al. are observed the increase in crystallization temperature and enthalpy of fusion and crystallization of extruded PET without using any chain extender while a reverse trend is observed by using PMDA.15
These observations were attributed to the lower crystallization rate of modified PET due to the restriction in the movement of the segments of branched chains folding in the lamellaes. It seems there is a threshold in branching level which under that limit, branches act as crystal defects and the crystallization rate decrease.14 Long chain branches, more than a certain value, could restrict chain mobility and lead to decrease in crystallinity.35
The crystallization and melting thermogram of the non-compatibilized and compatibilized PET/PP blends and their thermal characteristics are shown in Fig. 7, Fig. 8 and Table 5, respectively. The crystallization temperature of BPP showed a small increase from 119.7 °C to 121 °C in the blend that is due to the nucleating effect of MPET on BPP.
PP-g-MA is miscible with PP from one side, and moreover it's maleic anhydride groups can react with functional groups of MPET from other side. This compatibilizer, by coupling the BPP and MPET macromolecules, can restrict the mobility of chains, decrease the speed of nucleation and diffusion into the crystal cells39 and consequently leads to decrement in the crystallization temperature of BPP and MPET. The observed differences in the nucleating effect of dispersed particles could arise from different composition and/or different type of polymers. Heretofore, there is not available any publication on the BPP and chain extended/branched PET blends and their crystallization behaviour.
As is seen in Fig. 7, the crystallization peak of MPET transform to broad peaks around 200–205 °C by compatibilization. Bae et al. observed disappearance of the crystallization peak of PET in PET/PP (10/90) and PET/PP-g-HI (10/90, 30/70) blends. This behaviour was attributed to coincident crystallization of PET at crystallization temperature of PP-not PET PP co-crystallization40 and also decrease in the bulk of dispersed phase by compatibilization.41
The melting temperature of MPET in the blends is not changed, (Fig. 8) suggesting that the morphology of crystals are the same as neat component.42 The increase in crystallization temperature of neat PP or modified with peroxide is due to degradation of linear PP chains results in the enhanced mobility and arrangement of the molecular chains segments that favours the diffusion.41 Thermal properties changes are mostly based on the type and amount of peroxide, multifunctional monomers and/or co-agents, because they induced different reaction such as scission, branching and crosslinking which impact the polymer chains and eventually affect the crystallization and melting temperatures or enthalpies.
Conclusions
In this study PET and PP were chain extended and long chain branched to enhance their melt elongational strength and strain hardening behaviour that are the substantial properties in the processes such as foaming and thermoforming. The blends of BPP and chain extended long chain branched PET were prepared and the effect of PP-g-MA compatibilizer on the properties are investigated. The molecular architecture of modified PET and PP and their blends was evaluated by interpreting the rheological data provided by small amplitude oscillation rheometry and extensional rheology. This investigation leads to the following conclusions:The increase in molecular weight, widening of molecular weight distribution and branching in small extent are the consequence of PET modification. From other side, chain scission, narrower MWD and formation of long chain branched structures are the main phenomenon in branching of PP.
The strain hardening behaviour of PP melt in spite of decrease in molecular weight/zero shear viscosity reveals the formation of long chain branched structure.
With respect to strain hardening of MPET in elongational rheometry the creation of long chain branches simultaneously with chain extension is approved during the modification of PET with PMDA and PENTA.
The theoretical emulsion models are used to evaluate the effect of modification on the interfacial interaction of PET and PP in the blend. It is realized that these theoretical methods are not accurate enough to predict well the droplet-matrix interfacial interactions for PP/PET/PP-g-MA system.
The low crystallization rate of PET is improved by chain extension process and leads to enhanced crystallization rate as well as crystallization temperature.
References
- F. J. Stadler, J. Kaschta, H. Münstedt, F. Becker and M. Buback, Rheol. Acta, 2009, 48, 479 CrossRef CAS.
- C. Gabriel and H. Munstedt, J. Rheol., 2003, 47, 619 CrossRef CAS.
- R. P. Lagendijk, A. H. Hogt, A. Buijtenhuijs and A. D. Gotsis, Polymer, 2001, 42, 10035 CrossRef CAS.
- X. Wang, C. Tzoganakis and G. L. Rempel, J. Appl. Polym. Sci., 1996, 61, 1395 CrossRef CAS.
- A. J. DeNicola, J. W. Mayfield and B. J. Scheve, US Pat., 5731362, 1998.
- M. Ratzsch, J. Macromol. Sci., Part A: Pure Appl. Chem., 1999, 36, 1759 CrossRef PubMed.
- F. Ardakani, Y. Jahani and J. Morshedian, J. Appl. Polym. Sci., 2012, 125, 640 CrossRef CAS.
- F. Yoshii, K. Makuuchi, S. Kikukawa, T. Tanaka and J. Saitoh, J. Appl. Polym. Sci., 1996, 60, 617 CrossRef CAS.
- D. Graebling, Macromolecules, 2002, 35, 4602 CrossRef CAS.
- Z. Zhang, H. Xing, J. Qiu, Z. Jiang, H. Yu, X. Du, Y. Wang, L. Ma and T. Tang, Polymer, 2012, 53, 121 CrossRef CAS PubMed.
- P. Raffaa, M. B. Coltellia, S. Savia, S. Bianchia and V. Castelvetro, React. Funct. Polym., 2012, 72, 50 CrossRef PubMed.
- M. Härth, J. Kaschta and D. W. Schubert, Macromolecules, 2014, 47, 4471 CrossRef.
- J. S. Forsythe, K. Cheah, D. R. Nisbet, R. K. Gupta, A. Lau, A. R. Donovan, M. S. O'Shea and G. Moad, J. Appl. Polym. Sci., 2006, 100, 3646 CrossRef CAS.
- M. Xanthos, C. Wan, R. Dhavalikar, G. P. Karayannidis and D. N. Bikiaris, Polym. Int., 2004, 53, 1161 CrossRef CAS.
- P. Kiliaris, C. D. Papaspyrides and R. Pfaendner, J. Appl. Polym. Sci., 2002, 104, 1671 CrossRef.
- I. Xia, Z. Xi, T. Liu, X. Pan, C. Fan and L. Zhao, Polym. Eng. Sci., 2014 DOI:10.1002/pen.23995 , published online: 18 AUG.
- J. Song, R. H. Ewoldt, W. Hu, H. C. Silvis and C. W. Macosko, AIChE J., 2011, 57, 3496 CrossRef CAS.
- L. D'Orazio, R. Guarino, C. Manchrella, E. Martuscelli and G. Cecchin, J. Appl. Polym. Sci., 1997, 66, 2377 CrossRef.
- M. Pracella, D. Chionna, A. Pawlak and A. Galeski, J. Appl. Polym. Sci., 2005, 98, 2201 CrossRef CAS.
- M. Entezam, H. A. Khonakdar, A. A. Yousefi, S. H. Jafari and U. Wagenknecht, Macromol. Mater. Eng., 2012, 297, 312 CrossRef CAS.
- Y. X. Pang, D. M. Jia, H. J. Hu, D. J. Hourston and M. Song, Polymer, 2000, 41, 357 CrossRef CAS.
- M. F. Champagne, M. A. Huneault, C. Roux and W. Peyrel, Polym. Eng. Sci., 1999, 39, 976 CAS.
- J. Stange, C. Uhl and H. Münstedt, J. Rheol., 2005, 49, 1059 CrossRef CAS.
- D. Wan, L. Ma, Z. Zhang, H. Xing, L. Wang, Z. Jiang, G. Zhang and T. Tang, Polym. Degrad. Stab., 2012, 97, 40 CrossRef CAS PubMed.
- M. Gahleitner, Prog. Polym. Sci., 2001, 26, 895 CrossRef CAS.
- D. Auhl, J. Stange, H. Münstedt, B. Krause, D. Voigt, A. Lederer, U. Lappan and K. Lubkwitz, Macromolecules, 2004, 37, 9465 CrossRef CAS.
- M. K. Cheung and D. Chan, Polym. Int., 1997, 43, 281 CrossRef CAS.
- J. F. Palierne, Rheol. Acta, 1990, 29, 204 CrossRef CAS.
- M. Bousmina, Rheol. Acta, 1999, 38, 73 CrossRef CAS.
- D. Shi, G. H. Hu, Z. Ke, R. K. Y. Li and J. Yin, Polymer, 2006, 47, 4659 CrossRef CAS PubMed.
- F. H. Su and H. X. Huang, Polym. Eng. Sci., 2010, 50, 342 CAS.
- S. Li, M. Xiao, D. Wei, H. Xiao, F. Hu and A. Zheng, Polymer, 2009, 50, 6121 CrossRef CAS PubMed.
- A. Malmberg, C. Gabriel, T. Steffl and H. Münstedt, Macromolecules, 2002, 35, 1038 CrossRef CAS.
- X. D. Wang, Y. X. Zhang, B. G. Liu, Z. J. Du and H. Q. Li, Polym. J., 2008, 40, 450 CrossRef CAS.
- S. H. Tabatabaei, P. J. Carreau and A. Ajji, Chem. Eng. Sci., 2009, 64, 4719 CrossRef CAS PubMed.
- R. W. Campbell, US Pat., 4451606, 1984.
- K. Hirobe, T. Koyama, K. Matsumoto, M. Mihoichi, Y. Ohara and K. Mogamo, US Pat., 5886088, 1999.
- H. Y. Li, C. H. Lin, C. L. Wu and H. C. Kao, US Pat., 7879279, 2011.
- Y. Zhang, W. Guo, H. Zhang and C. Wu, Polym. Degrad. Stab., 2009, 94, 1135 CrossRef CAS PubMed.
- M. K. Bae, K. Y. Park, D. H. Kim and K. D. Suh, J. Appl. Polym. Sci., 2001, 81, 1056 CrossRef.
- F. H. Su and H. X. Huang, Polym. Eng. Sci., 2010, 50, 342 CAS.
- T. Hanley, D. Sutton, E. Heeley, G. Moad and R. B. Knott, J. Appl. Crystallogr., 2007, 40, 599 CrossRef.
| This journal is © The Royal Society of Chemistry 2015 |