Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Functionalization of photochromic dithienylmaleimides†
D.
Wutz
,
C.
Falenczyk
,
N.
Kuzmanovic
and
B.
König
*
Institute of Organic Chemistry, University of Regensburg, D-93051 Regensburg, Germany. E-mail: burkhard.koenig@ur.de
First published on 4th February 2015
Abstract
Photochromic dithienylmaleimides are well known molecular switches, but for applications the suitable functionalization of the photochromic scaffold is required. We report here synthetic routes to dithienylmaleimides, which are functionalized at three different positions: at each of the thiophene moieties and the maleimide nitrogen. A Perkin-type condensation of two thiophene precursors is used as the key step to assemble the maleimide core, which allows the synthesis of non-symmetrically substituted dithienylmaleimides, such as photochromic amino acids. A different approach to the maleimide core is provided by the reaction of a dithienylmaleic anhydride with amines or hydrazides leading to maleimide protected dithienylmaleimides and photochromic labeled natural amino acids. The photochromic properties of the new photoswitches were investigated showing reversible photochromism in polar organic solvents.
Introduction
Photochromism has attracted great attention in materials science1 and as a tool in molecular biology.2 A variety of applications are found in molecular optoelectronics3 and optical data storage.4–6 In the field of life sciences, molecular switches have been used to control enzyme activity,7–10 Watson–Crick base pairing,11–13 the regulation of neuronal activity by photochromic ligands for ion channels and receptors,14–20 antibiotic effects21,22 and even the agility of a living organism23 by light. This broad applicability is one of the reasons why photopharmacology has evolved into a vibrant field of research.24 Various photochromic molecules, like azobenzenes,25 spiropyrans,26 spirooxazines,26 fulgides27 and diarylethenes28,29 have been developed. All these photoswitches can be reversible toggled between two isomers using light. The well investigated dithienylethenes (DTEs), including dithienylmaleimides, are characterized by a nearly quantitative photochemical conversion between the photoisomers, which are often thermally stable. Irradiation with light of a specific wavelength switches the DTEs between their open and closed photoisomers, which differ in conformational flexibility and electronic conjugation (Fig. 1).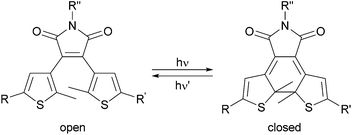 | ||
| Fig. 1 Reversible photochemical isomerization of a dithienylmaleimide between the open and closed photoisomer by irradiation with light of different wavelength. | ||
Many DTEs show high fatigue resistance.28 Despite their outstanding photophysical properties the synthesis of DTEs, in particular of non-symmetric derivatives, is laborious.13,30 Different synthetic routes for the preparation of dithienylmaleimides were established. Starting from 3,4-dibromomaleimides and 3,4-diiodomaleimides, respectively, both thiophene moieties can be attached by palladium catalyzed Suzuki coupling.31–33 However, only nitrogen protected maleimides can be used and the synthesis of non-symmetric compounds is challenging. Another route uses the reaction of a dithienylmaleic anhydride with amines to the corresponding maleimide.34–36 The synthesis of diarylmaleimides by intramolecular Perkin condensation of two independently prepared precursors gives selective access to non-symmetric diarylmaleimides.10,37,38 Compared to diarylperfluorocyclopentenes and diarylcyclopentenes, diarylmaleimides are more hydrophilic and better water soluble, which is valuable for applications in biology and pharmacy. The absorption maxima of diarylmaleimides are shifted to higher wavelengths and thus the photoisomerization can be induced by light with lower energy reducing potential cell damage.28 Moreover, the biocompatibility of diarylmaleimides is known from bisindolylmaleimides, for instance arcyriarubins and arcyriaflavins with antibiotic activities, several other potent protein kinase and sirtuin inhibitors.10,39–43 However, a better synthetic access to functionalized photochromic dithienylmaleimides is desirable in order to extend their applications. Herein we discuss the synthesis of functionalized dithienylmaleimides substituted on each thiophene moiety and the maleimide nitrogen atom.
Results and discussion
Synthesis
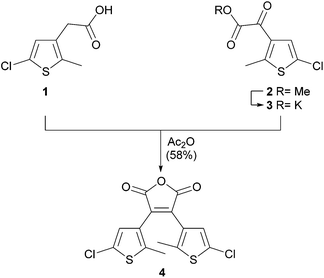
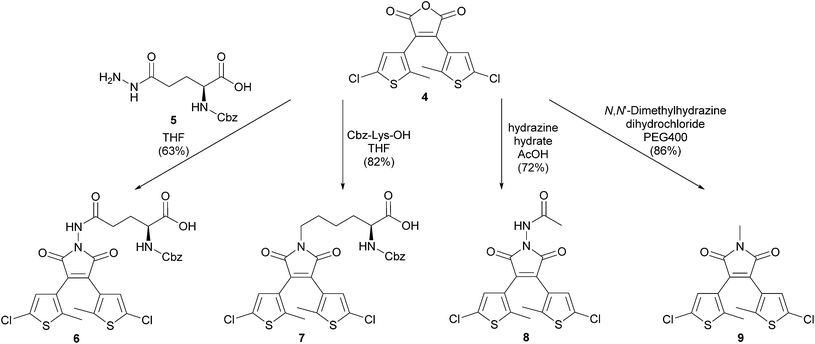
Methyl ester 2 was converted to its potassium salt 3 and condensed in a Perkin reaction with carboxylic acid 1 yielding the photochromic maleic anhydride 4. The anhydride moiety allows the subsequent functionalization with hydrazides or amines (Scheme 2). Therefore maleic anhydride 4 was treated with α-Cbz protected L-glutamic acid γ-hydrazide44 (5) and α-Cbz protected L-lysine to give amino acids 6 and 7 with a photochromic dithienylmaleimide on each sidechain. Photochromic tripeptides forming hydrogels with different aggregation modes mainly depending on the switch moiety were recently reported.45 The reaction of hydrazine hydrate in acetic acid as solvent and 1,2-dimethylhydrazine dihydrochloride, respectively, with maleic anhydride 4 afforded the maleimide nitrogen protected dithienylmaleimides 8 and 9 in good yields (Scheme 2). Remarkably, the formation of any maleic hydrazide or other tautomers was not observed. The protected maleimides 8 and 9 could be used for further functionalizations on the thiophene moieties by palladium-catalyzed cross coupling reactions or other reactions using the reactivity of the heteroaryl chlorides.
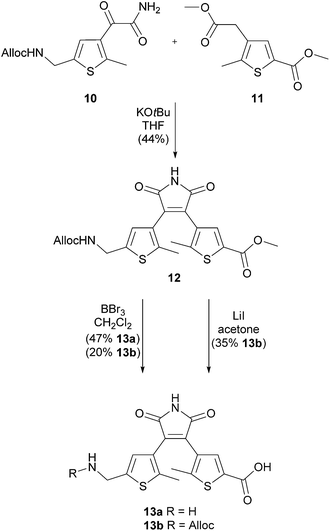 | ||
| Scheme 3 Perkin condensation of thiophenes 10 and 11 yielding dithienylmaleimide 12 and after deprotection 13a and 13b. | ||
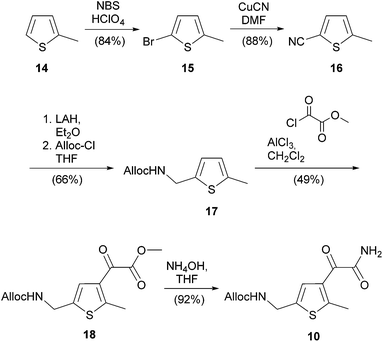
The reduction of nitrile 16 with lithium aluminum hydride followed by immediate protection with allyl chloroformate afforded carbamate 17 in good yield. Using Fmoc chloride instead led to the respective Fmoc derivative in lower yields and caused the formation of side products in the subsequent Friedel–Crafts acylation. The yield of glyoxylester 18 in the Friedel–Crafts acylation depends critically on the sequence of the reagent addition. Best results were obtained by mixing 17 and methyl chlorooxoacetate before adding aluminum chloride in small portions. Quenching the reaction with saturated sodium hydrogen carbonate solution avoids the addition of hydrochloric acid to the allyl double bond. Aminolysis with aqueous ammonia converted the glyoxylester 18 in high yield into compound 10. The overall yield for 10 after six steps is 22%.
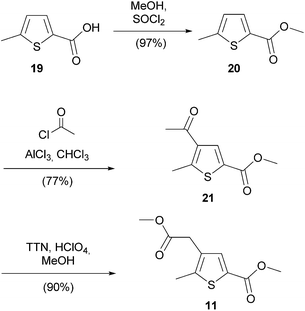
Thiophene 11 was prepared by esterification51 of methylthiophene acid 19 in the presence of thionyl chloride followed by Friedel–Crafts acylation and finally a thallium trinitrate (TTN) mediated oxidative rearrangement52 (Scheme 5). All intermediates were isolated in good to excellent yields with an overall yield of 68% for three steps. Initial moderate yields for the Friedel–Crafts acylation of around 40% significantly increased to 77% after rigorous removal of stabilizers from the solvent chloroform.
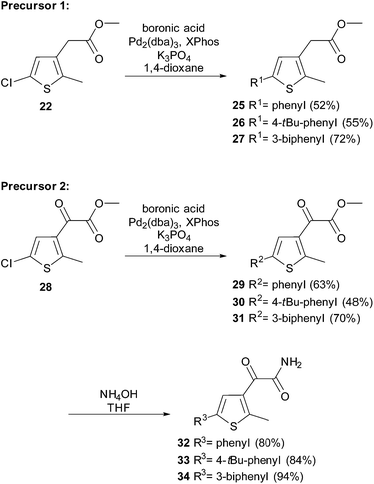
Recently, we described the synthesis of symmetric diarylmaleimides, with thiophene moieties functionalized by palladium-catalysis prior to the condensation reaction.10 Based on this strategy we prepared a small series of non-symmetric diarylmaleimides (Scheme 7).
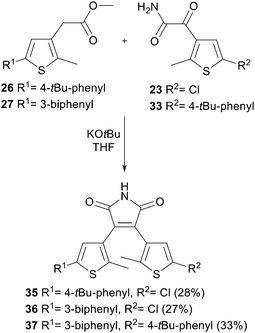
The Perkin condensation to the maleimide core was performed under basic conditions combining the different thiophenes. Scheme 8 summarizes the synthesis of the non-symmetric photoswitches 35–37.
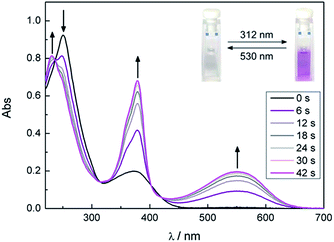
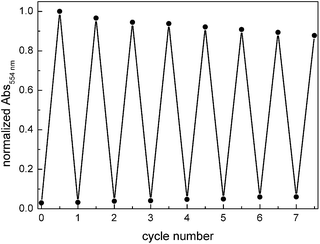
Upon irradiating a methanol solution of the ring-open form of compound 12 with UV light (312 nm), the absorption band at 250 nm immediately decreases. Simultaneously, new absorption maxima at 232 nm, 378 nm and 550 nm arise (Fig. 2) causing the color change of the sample from slightly yellow to purple. The isosbestic points indicate a clean conversion between two components. Compared to typical DTE-cyclopentenes the absorption maxima are red shifted. The photostationary state was reached after 42 s of irradiation (Herolab, 312 nm, 6 W) and the open form can be regained by irradiation with visible light (>420 nm) for 5 min. The photoswitchable amino acid 12 is stable over at least seven closing/opening cycles (Fig. 3).
The absorption maxima and their corresponding extinction coefficients for the open and closed form of all synthesized photochromic compounds are summarized in Table 1. Interestingly, the long wavelength absorption maximum of compound 13a is blue shifted to 537 nm compared to photoswitches 12 and 13b, which may indicate an interaction of the Alloc group with the dithienylmaleimide core. In contrast the selective removal of the methyl ester has almost no influence on the photochromic properties. In comparison to bischloro dithienylmaleimide 24 the functionalized maleimides 35–37 show a bathochromic shift in their absorption maxima of the closed photoisomer. The enlarged π-system of the substituted thiophenes can explain this shift to higher wavelengths.
| Compound | Solvent | Conc. [μM] | λ max open (ε) | λ max closed (ε) |
|---|---|---|---|---|
| a UV-Vis spectroscopic data are reported for solutions at 25 °C and reported in nm (λmax) and 103 cm−1 M−1 (ε). The PSS were obtained by irradiation of solutions of the open isomer with light of 312 nm (Herolab, 6 W). b Shoulder. | ||||
| 4 | MeOH | 100 | 242 (26.0), 298 (9.5) | 359 (16.0), 523 (3.7) |
| 6 | DMSO | 50 | 387 (4.6) | 359 (17.4), 510 (3.2) |
| 7 | DMSO | 50 | 381 (5.0) | 355 (20.8), 500 (4.1) |
| 8 | DMSO | 50 | 386 (6.0) | 359 (22.7), 508 (4.3) |
| 9 | DMSO | 50 | 380 (3.3) | 351 (12.9), 498 (2.7) |
| 12 | MeOH | 50 | 250 (18.5) | 232 (16.2), 378 (13.6), 550 (3.9) |
| 13a | MeOH | 50 | 252 (13.0) | 231 (11.3), 375 (10.0), 537 (2.7) |
| 13b | MeOH | 50 | 250 (14.3) | 232 (12.6), 378 (10.3), 549 (2.8) |
| 24 | MeOH | 50 | 240 (20.2), 370 (4.5) | 234 (20.6), 352 (13.8), 497 (2.5) |
| 35 | MeOH | 100 | 264 (18.5), 292 (17.1) | 369 (9.7), 543 (3.0) |
| 36 | MeOH | 100 | 255 (28.8), 291b (14.3) | 369 (10.1), 540 (3.7) |
| 37 | MeOH | 100 | 262 (26.1), 297 (20.7) | 391 (11.5), 586 (5.7) |
Conclusions
In summary, we have prepared several photochromic dithienylmaleimides. Maleimide nitrogen atom functionalized derivatives were obtained by the reaction of dithienylmaleic anhydride with different hydrazides and amines. Using a Perkin-type condensation non-symmetric dithienylmaleimides were synthesized including a photochromic amino acid and dithienylmaleimides with different aromatic substituents on each thiophene moiety. Reversible photoisomerization in dimethylsulfoxide and methanol was observed for all synthesized photochromic compounds.Experimental section
General information
Commercial reagents and starting materials were purchased from Acros Organics, Alpha-Aesar, Fluka, Sigma Aldrich or VWR and used without further purification. Solvents were used in p.a. quality and dried according to common procedures, if necessary. To purify the chloroform for Friedel–Crafts acylations, it was washed with sulfuric acid (1 M), dried over calcium chloride, filtered through silica, subsequently refluxed with phosphorus pentoxide (5–10 g L−1) and distilled under nitrogen atmosphere. Compounds 1,102,105,4415,4916,5020,5122,1025,1028,1029![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 32
10 and 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 were prepared according to previously reported procedures. Flash column chromatography was performed on a Biotage Isolera One automated flash purification system with UV/Vis detector using Sigma Aldrich MN silica gel 60 M (40–63 μm, 230–400 mesh) for normal phase or pre-packed Biotage SNAP cartridges (KP-C18-HS) for reversed phase chromatography. Reaction monitoring via TLC was performed on alumina plates coated with silica gel (Merck silica gel 60 F254, 0.2 mm). Melting points were determined using a Stanford Research Systems OptiMelt MPA 100. NMR spectra were recorded on a Bruker Avance 300 (1H 300.13 MHz, 13C 75.48 MHz), Bruker Avance 400 (1H 400.13 MHz, 13C 100.61 MHz) or Avance III 600 (1H 600.25 MHz, 13C 150.95 MHz) instrument. The spectra are referenced against the NMR-solvent, chemical shifts are reported in ppm and coupling constants J are given in Hz. Resonance multiplicity is abbreviated as: s (singlet), d (doublet), t (triplet), m (multiplet) and b (broad). Carbon NMR signals are reported with (+) for primary/tertiary, (−) for secondary and (q) for quaternary carbons. The assignment resulted from DEPT, HSQC and HMBC experiments. Mass spectra were recorded on a Finnigan MAT95 (EI-MS), Agilent Q-TOF 6540 UHD (ESI-MS, APCI-MS), Finnigan MAT SSQ 710 A (EI-MS, CI-MS) or ThermoQuest Finnigan TSQ 7000 (ES-MS, APCI-MS) spectrometer. UV/Vis absorption spectroscopy was performed using a Varian Cary BIO 50 UV/Vis/NIR spectrometer. IR-spectra were recorded with a Specac Golden Gate Diamond Single Reflection ATR System in a Bio-Rad FT-IR-Spectrometer Excalibur FTS 3000 and peak positions are reported in wavenumbers (cm−1). Standard hand-held lamps were used for visualizing TLC plates and to carry out the ring-closure reactions at 312 nm (Herolab, 312 nm, 6 W). The ring-opening reactions were performed with the light of a 200 W tungsten light bulb which was passed through a 420 nm cut-off filter to eliminate higher energy light or the light of a green LED (CREE-XP green, 530 nm, 700 mA). The power of the light is given based on the specifications supplied by the company when the lamps were purchased. A light detector was not used to measure the intensity during the irradiation experiments.
10 were prepared according to previously reported procedures. Flash column chromatography was performed on a Biotage Isolera One automated flash purification system with UV/Vis detector using Sigma Aldrich MN silica gel 60 M (40–63 μm, 230–400 mesh) for normal phase or pre-packed Biotage SNAP cartridges (KP-C18-HS) for reversed phase chromatography. Reaction monitoring via TLC was performed on alumina plates coated with silica gel (Merck silica gel 60 F254, 0.2 mm). Melting points were determined using a Stanford Research Systems OptiMelt MPA 100. NMR spectra were recorded on a Bruker Avance 300 (1H 300.13 MHz, 13C 75.48 MHz), Bruker Avance 400 (1H 400.13 MHz, 13C 100.61 MHz) or Avance III 600 (1H 600.25 MHz, 13C 150.95 MHz) instrument. The spectra are referenced against the NMR-solvent, chemical shifts are reported in ppm and coupling constants J are given in Hz. Resonance multiplicity is abbreviated as: s (singlet), d (doublet), t (triplet), m (multiplet) and b (broad). Carbon NMR signals are reported with (+) for primary/tertiary, (−) for secondary and (q) for quaternary carbons. The assignment resulted from DEPT, HSQC and HMBC experiments. Mass spectra were recorded on a Finnigan MAT95 (EI-MS), Agilent Q-TOF 6540 UHD (ESI-MS, APCI-MS), Finnigan MAT SSQ 710 A (EI-MS, CI-MS) or ThermoQuest Finnigan TSQ 7000 (ES-MS, APCI-MS) spectrometer. UV/Vis absorption spectroscopy was performed using a Varian Cary BIO 50 UV/Vis/NIR spectrometer. IR-spectra were recorded with a Specac Golden Gate Diamond Single Reflection ATR System in a Bio-Rad FT-IR-Spectrometer Excalibur FTS 3000 and peak positions are reported in wavenumbers (cm−1). Standard hand-held lamps were used for visualizing TLC plates and to carry out the ring-closure reactions at 312 nm (Herolab, 312 nm, 6 W). The ring-opening reactions were performed with the light of a 200 W tungsten light bulb which was passed through a 420 nm cut-off filter to eliminate higher energy light or the light of a green LED (CREE-XP green, 530 nm, 700 mA). The power of the light is given based on the specifications supplied by the company when the lamps were purchased. A light detector was not used to measure the intensity during the irradiation experiments.
3,4-Bis(5-chloro-2-methylthiophen-3-yl)furan-2,5-dione (4)
To obtain the potassium salt 3, the ester 2 was dissolved in EtOH (2.5 mL mmol−1) and KOH (1.0 eq.) was added. After stirring overnight the solvent was removed and the salt 3 was used without further purification. A mixture of the acid 1 (984 mg, 5.16 mmol), the potassium salt 3 (1.25 g, 5.16 mmol) and acetic anhydride (15 mL) was heated to 120 °C for 5 h. The reaction was cooled to room temperature and quenched by adding water (20 mL). The aqueous phase was extracted with EtOAc (3 × 15 mL). The combined organic phases were dried over MgSO4, filtered and the solvent was removed under reduced pressure. The crude product was purified by automated flash column chromatography (PE/CH2Cl2, 30–60% CH2Cl2) to yield the maleic anhydride 4 (1.07 g, 58%) as gray solid. Rf: 0.45 (PE/CH2Cl2: 1/1); m.p.: 205 °C; IR (neat) νmax: 3108, 2922, 1843, 1766, 1629, 1539, 1460, 1252, 1047, 1177, 990, 919; 1H-NMR (300 MHz, DMSO-d6): δ = 1.94 (s, 6H, thiophene-CH3), 7.03 (s, 2H, thiophene-H); 13C-NMR (75 MHz, DMSO-d6): δ = 14.1 (+), 125.2 (q), 125.3 (q), 127.2 (+), 134.5 (q), 141.4 (q), 164.5 (q); HRMS (ESI): calcd for C14H9Cl2O3S2 (M + H)+ 358.9365; found 358.9362.N 2-((Benzyloxy)carbonyl)-N5-(3,4-bis(5-chloro-2-methylthiophen-3-yl)-2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)-L-glutamine (6)
Maleic anhydride 4 (40 mg, 0.11 mmol) was added to a solution of acid hydrazide 5 (30 mg, 0.10 mmol) in THF (1 mL) in a crimp top vial. After heating to 85 °C for 16 h the reaction was quenched with 1 M aqueous HCl solution (1 mL) and water (1 mL). The aqueous phase was extracted with EtOAc (3 × 5 mL). The combined organic phases were dried over MgSO4 and the solvent was removed under reduced pressure. Purification by automated reversed phase flash column chromatography (MeCN/H2O with 0.05% TFA, 10–95% MeCN) yielded compound 6 (40 mg, 63%) as orange solid. Rf: 0.01 (EtOAc); m.p.: 103 °C; IR (neat) νmax: 3250, 2924, 1789, 1727, 1522, 1462, 1434, 1215, 1175, 990; 1H-NMR (300 MHz, DMSO-d6): δ = 1.74–1.88 (m, 1H, CO–(CH2)2–CH), 1.94 (s, 6H, thiophene-CH3), 2.00–2.10 (m, 1H, CO–(CH2)2–CH), 2.38–2.48 (m, 2H, CO–(CH2)2–CH), 4.03 (dt, J = 9.0, 4.8 Hz, 1H, CH2–CH–NH), 5.05 (s, 2H, O–CH2–Ph), 7.03 (s, 2H, thiophene-H), 7.31–7.40 (m, 5H, Ph-H), 7.68 (d, J = 8.0 Hz, 1H, CH–NH–CO), 10.63 (s, 1H, N–NH–CO), 12.70 (bs, 1H, COOH); 13C-NMR (75 MHz, DMSO-d6): δ = 14.2 (+), 26.1 (−), 29.1 (−), 53.1 (+), 65.4 (−), 125.0 (q), 125.8 (q), 127.6 (+), 127.6 (+), 127.7 (+), 128.3 (+), 131.2 (q), 136.8 (q), 140.6 (q), 156.1 (q), 166.9 (q), 170.8 (q), 173.4 (q); HRMS (ESI): calcd for C27H24Cl2N3O7S2 (M + H)+ 636.0427; found 636.0428.(S)-2-(((Benzyloxy)carbonyl)amino)-6-(3,4-bis(5-chloro-2-methylthiophen-3-yl)-2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)hexanoic acid (7)
Triethylamine (131 μL, 0.95 mmol) was added to a suspension of maleic anhydride 4 (97 mg, 0.27 mmol) and Cbz-Lys-OH (83 mg, 0.30 mmol) in THF (5 mL) in a crimp top vial. After heating to 85 °C for 16 h the reaction was quenched with 1 M aqueous HCl solution (3 mL) and water (3 mL). The aqueous phase was extracted with EtOAc (3 × 15 mL). The combined organic phases were dried over MgSO4 and the solvent was removed under reduced pressure. Purification by automated reversed phase flash column chromatography (MeCN/H2O with 0.05% TFA, 50–100% MeCN) yielded compound 7 (138 mg, 82%) as orange solid. Rf: 0.09 (PE/EtOAc: 1/1); m.p.: 88 °C; IR (neat) νmax: 3351, 3095, 2932, 2870, 1698, 1526, 1438, 1404, 1211, 989; 1H-NMR (400 MHz, DMSO-d6): δ = 1.29–1.43 (m, 2H, CH2–(CH2)3–CH), 1.51–1.65 (m, 3H, CH2–(CH2)3–CH), 1.67–1.79 (m, 1H, CH2–(CH2)3–CH), 1.91 (s, 6H, thiophene-CH3), 3.49 (t, J = 7.1 Hz, 2H, N–CH2–(CH2)3), 3.93 (dt, J = 7.9, 4.6 Hz, 1H, NH–CH–CH2), 5.02 (s, 2H, O–CH2–Ph), 7.00 (s, 2H, thiophene-H), 7.28–7.37 (m, 5H, Ph-H), 7.57 (d, J = 8.0 Hz, 1H, CO–NH–CH), 12.55 (s, 1H, COOH); 13C-NMR (101 MHz, DMSO-d6): δ = 14.1 (+), 22.8 (−), 27.4 (−), 30.2 (−), 37.7 (−), 53.5 (+), 65.3 (−), 124.6 (q), 126.4 (q), 127.6 (+), 127.7 (+), 127.7 (+), 128.2 (+), 132.3 (q), 136.9 (q), 139.8 (q), 169.6 (q), 173.8 (q); HRMS (ESI): calcd for C28H27Cl2N2O6S2 (M + H)+ 621.0682; found 621.0684.N-(3,4-Bis(5-chloro-2-methylthiophen-3-yl)-2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)acetamide (8)
Hydrazine hydrate (39 μL, 0.81 mmol) was added to a solution of maleic anhydride 4 (97 mg, 0.27 mmol) in acetic acid (3.5 mL). The reaction mixture was heated to 100 °C for 20 h and then water (10 mL) was added. The aqueous phase was extracted with EtOAc (3 × 10 mL). The combined organic phases were dried over MgSO4 and the solvent was evaporated in vacuo. Purification by automated flash column chromatography (PE/EtOAc, 15–50% EtOAc) yielded compound 8 (81 mg, 72%) as orange solid. Rf: 0.23 (PE/EtOAc: 2/1); m.p.: 131 °C; IR (neat) νmax: 3328, 3088, 2924, 2359, 1717, 1702, 1510, 1429, 1255, 1193, 988; 1H-NMR (400 MHz, DMSO-d6): δ = 1.95 (s, 6H, thiophene-CH3), 2.03 (s, 3H, CO–CH3), 7.01 (s, 2H, thiophene-H), 10.58 (s, 1H, NH); 13C-NMR (101 MHz, DMSO-d6): δ = 14.2 (+), 20.0 (+), 125.0 (q), 125.8 (q), 127.6 (+), 131.3 (q), 140.6 (q), 166.9 (q), 168.5 (q); HRMS (ESI): calcd for C16H13Cl2N2O3S2 (M + H)+ 414.9739; found 414.9741.3,4-Bis(5-chloro-2-methylthiophen-3-yl)-1-methyl-1H-pyrrole-2,5-dione (9)
Maleic anhydride 4 (54 mg, 0.15 mmol) and 1,2-dimethylhydrazine dihydrochloride (60 mg, 0.45 mmol) were heated to 160 °C for 12 h in PEG400 (2 mL) in a crimp top vial. Then water (15 mL) was added and the aqueous phase was extracted with EtOAc (3 × 15 mL). The combined organic phases were washed with brine (50 mL), dried over MgSO4 and the solvent was removed under reduced pressure. Purification by automated flash column chromatography (PE/EtOAc, 3–10% EtOAc) yielded compound 9 (48 mg, 86%) as orange foam. Rf: 0.32 (PE/EtOAc: 19/1); IR (neat) νmax: 3098, 2924, 2851, 1765, 1697, 1435, 1386, 1250, 1174, 980; 1H-NMR (300 MHz, CDCl3): δ = 1.93 (s, 6H, thiophene-CH3), 3.13 (s, 3H, N–CH3), 6.89 (s, 2H, thiophene-H); 13C-NMR (75 MHz, CDCl3): δ = 14.9 (+), 24.4 (+), 126.0 (q), 127.2 (+), 127.2 (q), 132.7 (q), 140.2 (q), 170.2 (q); HRMS (ESI): calcd for C15H12Cl2NO2S2 (M + H)+ 373.9649; found 373.9652.Allyl ((4-(2-amino-2-oxoacetyl)-5-methylthiophen-2-yl)methyl)carbamate (10)
To a solution of oxoacetate 18 (282 mg, 0.95 mmol) in THF (5 mL) was added a NH4OH solution (32% in H2O) (1.18 mL, 9.50 mmol) at 0 °C. The reaction was stirred for 90 min at room temperature and then quenched with water (5 mL). The aqueous phase was extracted with EtOAc (3 × 10 mL). The combined organic phases were dried over MgSO4 and the solvent was removed under reduced pressure. Compound 10 (253 mg, 94%) was obtained as yellow solid and used without further purification. Rf: 0.21 (PE/EtOAc: 1/1); m.p.: 108 °C; IR (neat) νmax: 3402, 3301, 3167, 2962, 1750, 1686, 1649, 1535, 1460, 1254, 1047, 796; 1H-NMR (400 MHz, CDCl3): δ = 2.70 (s, 3H, thiophene-CH3), 4.44 (d, J = 6.1 Hz, 2H, thiophene-CH2NH), 4.59 (d, J = 5.1 Hz, 2H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2O), 5.21 (dd, J = 10.4, 0.5 Hz, 1H, CH2
CHCH2O), 5.21 (dd, J = 10.4, 0.5 Hz, 1H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2), 5.24–5.43 (m, 2H, CH2
CHCH2), 5.24–5.43 (m, 2H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2 and NH), 5.90 (ddt, J = 16.2, 10.7, 5.5 Hz, 1H, CH2
CHCH2 and NH), 5.90 (ddt, J = 16.2, 10.7, 5.5 Hz, 1H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2), 6.05 (bs, 1H, NH2), 7.06 (bs, 1H, NH2), 7.86 (s, 1H, thiophene-H); 13C-NMR (101 MHz, CDCl3): δ = 16.7 (+), 39.8 (−), 65.9 (−), 117.9 (−), 129.0 (+), 130.8 (q), 132.7 (+), 137.0 (q), 155.7 (q), 156.1 (q), 164.4 (q), 182.1 (q); HRMS (ESI): calcd for C12H18N3O4S (M + NH4)+ 300.1013; found 300.1012.
CHCH2), 6.05 (bs, 1H, NH2), 7.06 (bs, 1H, NH2), 7.86 (s, 1H, thiophene-H); 13C-NMR (101 MHz, CDCl3): δ = 16.7 (+), 39.8 (−), 65.9 (−), 117.9 (−), 129.0 (+), 130.8 (q), 132.7 (+), 137.0 (q), 155.7 (q), 156.1 (q), 164.4 (q), 182.1 (q); HRMS (ESI): calcd for C12H18N3O4S (M + NH4)+ 300.1013; found 300.1012.
Methyl 4-(2-methoxy-2-oxoethyl)-5-methylthiophene-2-carboxylate (11)
Thallium trinitrate (850 mg, 1.91 mmol) and 70% HClO4 (0.30 mL) were added to a suspension of 21 (316 mg, 1.59 mmol) in MeOH (10 mL) at room temperature. After stirring for 24 h the mixture was concentrated under vacuum and diluted with water (10 mL). The aqueous phase was extracted with EtOAc (3 × 10 mL) and the combined organic layers were dried over MgSO4. The solvent was evaporated and purification of the crude product by automated flash column chromatography (PE/EtOAc, 3–15% EtOAc) yielded compound 11 (331 mg, 91%) as colorless oil. Rf: 0.34 (PE/EtOAc: 5/1); IR (neat) νmax: 2997, 2953, 2845, 1736, 1704, 1535, 1457, 1392, 1331, 1291, 1250, 1194, 1132, 1063, 1006, 927, 874, 785, 751; 1H-NMR (400 MHz, CDCl3): δ = 2.42 (s, 3H, thiophene-CH3), 3.54 (s, 2H, thiophene-CH2C(O)OCH3), 3.69 (s, 3H, thiophene-CH2C(O)OCH3), 3.84 (s, 3H, C(O)OCH3), 7.60 (s, 1H, thiophene-H); 13C-NMR (101 MHz, CDCl3): δ = 13.9 (+), 33.9 (−), 52.1 (+), 52.3 (+), 129.2 (q), 130.8 (q), 135.7 (+), 144.1 (q), 162.7 (q), 171.0 (q); HRMS (APCI): calcd for C10H12O4S (M + H)+ 229.0529; found 229.0531.Methyl 4-(4-(5-((((allyloxy)carbonyl)amino)methyl)-2-methyl-thiophen-3-yl)-2,5-dioxo-2,5-dihydro-1H-pyrrol-3-yl)-5-methylthiophene-2-carboxylate (12)
KOtBu (1 M in THF, 0.88 mL, 0.88 mmol) was added to a solution of glyoxylamide 10 (206 mg, 0.73 mmol) in anhydrous THF (5 mL) at 0 °C under nitrogen atmosphere. After stirring for 90 min at 0 °C, diester 11 (200 mg, 0.88 mmol) in THF (2 mL) was added at 0 °C and stirred for 3 days at room temperature. Then the reaction was quenched with 1 M aqueous HCl solution (3 mL) and diluted with EtOAc (10 mL). The organic phase was washed with water (2 × 10 mL), brine (10 mL) and dried over MgSO4. The solvent was removed under reduced pressure and purification of the crude product by automated flash column chromatography (PE/EtOAc, 25–50% EtOAc) yielded 12 (148 mg, 44%) as yellow foam. Rf: 0.20 (PE/EtOAc: 2/1); IR (neat) νmax: 3289, 3070, 2952, 1703, 1540, 1458, 1339, 1248, 994, 909, 727; 1H-NMR (400 MHz, CDCl3): δ = 1.90 (s, 3H, thiophene-CH3); 1.98 (s, 3H, thiophene-CH3), 3.87 (s, 1H, OCH3), 4.45 (d, J = 6.0 Hz, 2H, thiophene-CH2NH), 4.60 (d, J = 4.9 Hz, 2H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2O), 5.13–5.27 (m, 2H, CH2
CHCH2O), 5.13–5.27 (m, 2H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2 and CH2NHCO), 5.31 (dd, J = 17.2, 1.2 Hz, 1H, CH2
CHCH2 and CH2NHCO), 5.31 (dd, J = 17.2, 1.2 Hz, 1H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2), 5.92 (ddt, J = 16.2, 10.8, 5.5 Hz, 1H, CH2
CHCH2), 5.92 (ddt, J = 16.2, 10.8, 5.5 Hz, 1H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2), 6.90 (s, 1H, thiophene-H), 7.74 (s, 1H, thiophene-H), 8.03 (bs, 1H, CONHCO); 13C-NMR (101 MHz, CDCl3): δ = 15.0 (+), 15.3 (+), 39.9 (−), 52.3 (+), 65.9 (−), 117.9 (−), 125.8 (q), 126.7 (+), 127.5 (q), 130.9 (q), 132.7 (+), 134.8 (q), 134.9 (+), 139.4 (q), 142.1 (q), 148.6 (q), 156.0 (q), 162.1 (q), 170.0 (q), 170.2 (q); HRMS (ESI): calcd for C21H21N2O6S2 (M + H)+ 461.0838; found 461.0836.
CHCH2), 6.90 (s, 1H, thiophene-H), 7.74 (s, 1H, thiophene-H), 8.03 (bs, 1H, CONHCO); 13C-NMR (101 MHz, CDCl3): δ = 15.0 (+), 15.3 (+), 39.9 (−), 52.3 (+), 65.9 (−), 117.9 (−), 125.8 (q), 126.7 (+), 127.5 (q), 130.9 (q), 132.7 (+), 134.8 (q), 134.9 (+), 139.4 (q), 142.1 (q), 148.6 (q), 156.0 (q), 162.1 (q), 170.0 (q), 170.2 (q); HRMS (ESI): calcd for C21H21N2O6S2 (M + H)+ 461.0838; found 461.0836.
4-(4-(5-(Aminomethyl)-2-methylthiophen-3-yl)-2,5-dioxo-2,5-dihydro-1H-pyrrol-3-yl)-5-methylthiophene-2-carboxylic acid (13a) and 4-(4-(5-((((allyloxy)carbonyl)amino)methyl)-2-methylthiophen-3-yl)-2,5-dioxo-2,5-dihydro-1H-pyrrol-3-yl)-5-methylthiophene-2-carboxylic acid (13b)
A solution of BBr3 (1 M in CH2Cl2, 2.0 mL, 2.00 mmol) was added to a solution of compound 12 (92 mg, 0.20 mmol) in anhydrous CH2Cl2 (6 mL) in a crimp top vial. The mixture was heated to 40 °C for 5 h. Then water (4 mL) was added via syringe and the suspension was stirred at 40 °C for additional 30 min. After cooling to room temperature the solvent was removed at the rotary evaporator. Purification by automated reversed phase flash column chromatography (MeCN/H2O with 0.05% TFA, 3–100% MeCN) yielded compound 13a (34 mg, 47%) as yellow solid and compound 13b (18 mg, 20%) as yellow solid.Analytical data of 13a
R f: 0.02 (PE/EtOAc: 1/1); m.p.: 173 °C; IR (neat) νmax: 3008, 2924, 1766, 1712, 1681, 1545, 1463, 1344, 1188, 1137, 1001, 839, 799, 756, 723; 1H-NMR (600 MHz, MeOD): δ = 2.00 (s, 3H, thiophene-CH3), 2.08 (s, 3H, thiophene-CH3), 4.27 (s, 2H, thiophene-CH2NH), 7.18 (s, 1H, thiophene-H), 7.64 (s, 1H, thiophene-H); 13C-NMR (151 MHz, MeOD): δ = 14.7 (+), 15.3 (+), 38.5 (−), 128.5 (q), 129.3 (q), 132.2 (+), 132.8 (q), 133.1 (q), 135.0 (q), 135.7 (q), 136.3 (+), 144.6 (q), 149.7 (q), 164.6 (q), 172.4 (q), 172.6 (q); HRMS (ESI): calcd for C16H15N2O4S2 (M + H)+ 363.0469; found 363.0468.Analytical data of 13b
R f: 0.04 (PE/EtOAc: 1/1); m.p.: 94 °C; IR (neat) νmax: 2926, 1981, 1769, 1709, 1544, 1459, 1344, 1246, 1185, 1150, 1049, 991, 849, 762; 1H-NMR (300 MHz, CD3CN): δ = 1.93 (s, 3H, thiophene-CH3), 1.97 (s, 3H, thiophene-CH3), 4.33 (d, J = 6.3 Hz, 2H, thiophene-CH2NH), 4.52 (d, J = 5.3 Hz, 2H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2O), 5.18 (dd, J = 10.5, 1.4 Hz, 1H, CH2
CHCH2O), 5.18 (dd, J = 10.5, 1.4 Hz, 1H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2), 5.27 (dd, J = 17.3, 1.6 Hz, 1H, CH2
CHCH2), 5.27 (dd, J = 17.3, 1.6 Hz, 1H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2), 5.74–6.05 (m, 1H, CH2
CHCH2), 5.74–6.05 (m, 1H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2), 6.14 (bs, 1H, CH2NHCO), 6.79 (s, 1H, thiophene-H), 7.60 (s, 1H, thiophene-H), 8.80 (bs, 1H, COOH); 13C-NMR (75 MHz, CD3CN): δ = 14.8 (+), 15.2 (+), 40.1 (−), 66.0 (−), 117.5 (−), 127.2 (q), 127.4 (+), 129.1 (q), 131.4 (q), 134.1 (q), 134.4 (+), 136.2 (+), 136.2 (q), 141.2 (q), 141.8 (q), 149.6 (q), 157.1 (q), 162.9 (q), 171.6 (q), 171.7 (q); HRMS (ESI): calcd for C20H18N2O6S2 (M + H)+ 447.0679; found 447.0676.
CHCH2), 6.14 (bs, 1H, CH2NHCO), 6.79 (s, 1H, thiophene-H), 7.60 (s, 1H, thiophene-H), 8.80 (bs, 1H, COOH); 13C-NMR (75 MHz, CD3CN): δ = 14.8 (+), 15.2 (+), 40.1 (−), 66.0 (−), 117.5 (−), 127.2 (q), 127.4 (+), 129.1 (q), 131.4 (q), 134.1 (q), 134.4 (+), 136.2 (+), 136.2 (q), 141.2 (q), 141.8 (q), 149.6 (q), 157.1 (q), 162.9 (q), 171.6 (q), 171.7 (q); HRMS (ESI): calcd for C20H18N2O6S2 (M + H)+ 447.0679; found 447.0676.
Alternative procedure to obtain 13b
Compound 12 (40 mg, 0.09 mmol) was dissolved in acetone (10 mL) and LiI (350 mg, 2.60 mmol) was added. The mixture was heated to 100 °C overnight. After cooling to room temperature it was quenched with 1 M aqueous HCl solution (5 mL) and diluted with CH2Cl2 (5 mL). The phases were separated and the aqueous phase was extracted with CH2Cl2 (3 × 5 mL). The combined organic phases were dried over Na2SO4 and the solvent was removed at the rotary evaporator. Automated reversed phase flash column chromatography (MeCN/H2O with 0.05% TFA, 3–100% MeCN) yielded compound 13b (14 mg, 35%) as yellow solid.Allyl ((5-methylthiophen-2-yl)methyl)carbamate (17)
LAH (2.78 g, 73.2 mmol) was added in portions to a solution of nitrile 16 (3.01 g, 24.4 mmol) in anhydrous Et2O (250 mL) at 0 °C under nitrogen atmosphere. After stirring for 4 h at room temperature the reaction was quenched with water (80 mL) and saturated aqueous NaHCO3 solution (50 mL) at 0 °C. The suspension was filtered and the aqueous phase was extracted with Et2O (3 × 80 mL). The combined organic phases were dried over MgSO4 and concentrated in vacuo. Then the residue was dissolved in anhydrous THF (100 mL) and pyridine (2.47 mL, 30.50 mmol) was added at 0 °C. Within 1 h allyl chloroformate (4.02 mL, 37.82 mmol) in anhydrous THF (5 mL) was dropped to the solution via a syringe pump at 0 °C. After stirring for 14 h at room temperature the reaction was quenched cautiously with water (50 mL) and extracted with EtOAc (3 × 30 mL). The combined organic phases were dried over MgSO4 and the solvent was removed under reduced pressure. Purification of the crude product by automated flash column chromatography (PE/EtOAc, 8–15% EtOAc) yielded 17 (3.40 g, 66%) as yellow oil. Rf: 0.20 (PE/EtOAc: 9/1); IR (neat) νmax: 3335, 3073, 2922, 1695, 1514, 1426, 1236, 982, 799; 1H-NMR (400 MHz, CDCl3): δ = 2.44 (s, 3H, thiophene-CH3), 4.44 (d, J = 5.7 Hz, 2H, thiophene-CH2NH), 4.61 (d, J = 5.3 Hz, 2H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2O), 5.05 (bs, 1H, NH), 5.25 (dd, J = 10.4, 0.3 Hz, 1H, CH2
CHCH2O), 5.05 (bs, 1H, NH), 5.25 (dd, J = 10.4, 0.3 Hz, 1H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2), 5.30 (dd, J = 17.1, 1.2 Hz, 1H, CH2
CHCH2), 5.30 (dd, J = 17.1, 1.2 Hz, 1H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2), 5.92 (ddt, J = 16.4, 10.8, 5.7 Hz, 1H, CH2
CHCH2), 5.92 (ddt, J = 16.4, 10.8, 5.7 Hz, 1H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2), 6.57 (dd, J = 3.1, 1.0 Hz, 1H, 4-thiophene-H), 6.74 (d, J = 3.1 Hz, 1H, 3-thiophene-H); 13C-NMR (101 MHz, CDCl3): δ = 15.4 (+), 40.1 (−), 65.7 (−), 117.8 (−), 124.8 (+), 125.7 (+), 132.8 (+), 138.8 (q), 139.9 (q), 155.9 (q); HRMS (ESI): calcd for C10H14NO2S (M + H)+ 212.0740; found 212.0740.
CHCH2), 6.57 (dd, J = 3.1, 1.0 Hz, 1H, 4-thiophene-H), 6.74 (d, J = 3.1 Hz, 1H, 3-thiophene-H); 13C-NMR (101 MHz, CDCl3): δ = 15.4 (+), 40.1 (−), 65.7 (−), 117.8 (−), 124.8 (+), 125.7 (+), 132.8 (+), 138.8 (q), 139.9 (q), 155.9 (q); HRMS (ESI): calcd for C10H14NO2S (M + H)+ 212.0740; found 212.0740.
Methyl 2-(5-((((allyloxy)carbonyl)amino)methyl)-2-methylthiophen-3-yl)-2-oxoacetate (18)
Carbamate 17 (169 mg, 0.80 mmol) and methyl chlorooxoacetate (81 μL, 0.88 mmol) were dissolved in anhydrous CH2Cl2 (6 mL) under nitrogen atmosphere. Then aluminum chloride (427 mg, 3.20 mmol) was added in portions at 0 °C and the suspension was stirred for 20 h at room temperature. The reaction was quenched with saturated aqueous NaHCO3 solution (1 mL) at 0 °C and diluted with water (5 mL). The aqueous phase was extracted with CH2Cl2 (3 × 5 mL), the combined organic layers were washed with brine (10 mL) and dried over MgSO4. After evaporation of the solvent the crude product was purified by automated flash column chromatography (PE/EtOAc, 15–40% EtOAc) to obtain 18 (117 mg, 49%) as brown oil. Rf: 0.22 (PE/EtOAc: 3/1); IR (neat) νmax: 3395, 2954, 1726, 1670, 1517, 1434, 1242, 1200, 1112, 984, 757; 1H-NMR (400 MHz, CDCl3): δ = 2.70 (s, 3H, thiophene-CH3), 3.91 (s, 3H, OCH3), 4.42 (d, J = 6.1 Hz, 2H, thiophene-CH2NH), 4.58 (d, J = 5.3 Hz, 2H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2O), 5.16–5.34 (m, 3H, CH2
CHCH2O), 5.16–5.34 (m, 3H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2 and NH), 5.90 (ddt, J = 16.3, 10.8, 5.6 Hz, 1H, CH2
CHCH2 and NH), 5.90 (ddt, J = 16.3, 10.8, 5.6 Hz, 1H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2), 7.32 (s, 1H, thiophene-H); 13C-NMR (101 MHz, CDCl3): δ = 16.3 (+), 39.7 (−), 52.8 (+), 65.9 (−), 118.0 (−), 127.5 (+), 131.0 (q), 132.6 (+), 138.0 (q), 154.8 (q), 156.1 (q), 164.0 (q), 180.0 (q); HRMS (ESI): calcd for C13H16NO5S (M + H)+ 298.0744; found 298.0744.
CHCH2), 7.32 (s, 1H, thiophene-H); 13C-NMR (101 MHz, CDCl3): δ = 16.3 (+), 39.7 (−), 52.8 (+), 65.9 (−), 118.0 (−), 127.5 (+), 131.0 (q), 132.6 (+), 138.0 (q), 154.8 (q), 156.1 (q), 164.0 (q), 180.0 (q); HRMS (ESI): calcd for C13H16NO5S (M + H)+ 298.0744; found 298.0744.
Methyl 4-acetyl-5-methylthiophene-2-carboxylate (21)
Thiophene 20 (800 mg, 5.12 mmol) and acetyl chloride (550 μL, 7.68 mmol) were dissolved in purified anhydrous CHCl3 (10 mL) under nitrogen atmosphere. After cooling to 0 °C aluminum chloride (2.05 g, 15.4 mmol) was added in small portions. The yellow suspension was heated to 45 °C overnight upon turning bright red, then the reaction was quenched with ice/water and the aqueous phase was extracted with EtOAc (3 × 10 mL). The combined organic phases were washed with a saturated aqueous solution of NaHCO3 (10 mL) and brine (10 mL). The organic phase was dried over MgSO4 and the solvent was evaporated. The crude product was purified by automated flash column chromatography (PE/EtOAc, 5–25% EtOAc) and compound 21 (781 mg, 77%) was obtained as colorless solid. Rf: 0.41 (PE/EtOAc: 3/1); m.p.: 84 °C; IR (neat) νmax: 3007, 2957, 1717, 1678, 1539, 1457, 1439, 1254, 1233, 1074, 1021, 745; 1H-NMR (300 MHz, CDCl3): δ = 2.52 (s, 3H, thiophene-CH3), 2.76 (s, 3H, acetyl-CH3), 3.88 (s, 3H, OCH3), 8.03 (s, 1H, thiophene-H); 13C-NMR (75 MHz, CDCl3): δ = 16.8 (+), 29.6 (+), 52.3 (+), 128.5 (q), 135.0 (+), 136.3 (q), 155.8 (q), 162.0 (q), 193.7 (q); HRMS (APCI): calcd for C9H10O3S (M + H)+ 199.0423; found 199.0424.General procedure A: Suzuki coupling
To a suspension of Pd2(dba)3 (5 mol%), XPhos (10 mol%), the appropriate boronic acid (1.5 eq.) and K3PO4 (1.5 eq.) in 1,4-dioxane (0.5 M) the appropriate ester (1.0 eq.) was added. The resulting mixture was heated to 100 °C and stirred overnight. After cooling to room temperature the reaction mixture was diluted with EtOAc and the organic phase was washed two times with water. The organic phase was dried over MgSO4, filtered and the solvent was removed under reduced pressure.General procedure B: aminolysis
An NH4OH solution (25% in H2O) (10.0 eq.) was added to a solution of the appropriate oxoacetate (1.0 eq.) in THF (0.3 M) at 0 °C. The reaction was stirred for 1 h at room temperature and then quenched with water. The aqueous phase was extracted with EtOAc. The combined organic phases were dried over MgSO4, filtered and the solvent was removed under reduced pressure.General procedure C: Perkin condensation
KOtBu (1 M in THF) (1.2 eq.) was added to a solution of the appropriate amide (1.0 eq.) in THF (0.2 M) at 0 °C. After 90 min stirring at 0 °C the appropriate ester (1.0 eq.) was added at 0 °C and stirred overnight at room temperature. The reaction was quenched with 1 M HCl and diluted with EtOAc. The organic phase was washed three times with water and one time with brine. The organic phase was dried over MgSO4, filtered and the solvent was removed under reduced pressure.2-(5-Chloro-2-methylthiophen-3-yl)-2-oxoacetamide (23)
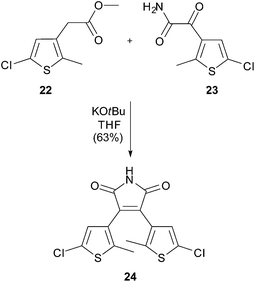
Compound 23 was prepared from 28 (800 mg, 3.66 mmol) according to general procedure B. The amide 23 (640 mg, 85%) was obtained as light yellow solid and used without further purification. m.p.: 183 °C; IR (neat) νmax: 3446, 3252, 1996, 1670, 1618, 1296, 1221, 1153; 1H-NMR (300 MHz, DMSO-d6): δ = 2.64 (s, 3H, thiophene-CH3), 7.49 (s, 1H, thiophene-H), 7.94 (bs, 1H, NH), 8.25 (bs, 1H, NH); 13C-NMR (75 MHz, DMSO-d6): δ = 15.2 (+), 123.9 (q), 128.4 (+), 131.4 (q), 150.9 (q), 166.1 (q), 184.2 (q); HRMS (ESI): calcd for C7H10ClN2O2S (M + NH4)+ 221.0146; found 221.0144.3,4-Bis(5-chloro-2-methylthiophen-3-yl)-1H-pyrrole-2,5-dione (24)
Compound 24 was prepared from amide 23 (600 mg, 2.95 mmol) and ester 22 (720 mg, 3.54 mmol) according to general procedure C. Purification by automated flash column chromatography (heptane/EtOAc: 5/1) yielded 24 (660 mg, 63%) as orange solid. Rf: 0.18 (heptane/EtOAc: 5/1); m.p.: 237 °C; IR (neat) νmax: 3381, 2939, 2818, 1653, 1437, 1002; 1H-NMR (400 MHz, DMSO-d6): δ = 1.87 (s, 6H, thiophene-CH3), 6.97 (s, 2H, thiophene-H), 11.25 (bs, 1H, NH); 13C-NMR (101 MHz, DMSO-d6): δ = 14.6 (+), 125.0 (q), 127.0 (q), 128.4 (+), 133.6 (q), 140.0 (q), 171.4 (q); HRMS (ESI): calcd for C14H10Cl2NO2S2 (M + H)+ 357.9525; found 357.9523.Methyl 2-(5-(4-(tert-butyl)phenyl)-2-methylthiophen-3-yl)acetate (26)
Compound 26 was prepared from 22 (200 mg, 0.98 mmol) according to general procedure A. Purification by automated flash column chromatography (PE/EtOAc, 0–25% EtOAc) yielded 26 (163 mg, 55%) as yellow liquid. Rf: 0.63 (PE/EtOAc: 5/1); IR (neat) νmax: 2961, 1736, 1609, 1520, 1435, 1364, 1239, 1018, 825; 1H-NMR (300 MHz, CDCl3): δ = 1.33 (s, 9H, tBu), 2.41 (s, 3H, thiophene-CH3), 3.55 (s, 2H, thiophene-CH2C(O)OCH3), 3.71 (s, 3H, thiophene-CH2C(O)OCH3), 7.08 (s, 1H, thiophene-H), 7.32–7.40 (m, 2H, Ph), 7.43–7.52 (m, 2H, Ph); 13C-NMR (75 MHz, CDCl3): δ = 13.3 (+), 31.3 (+), 34.1 (−), 34.6 (q), 52.1 (+), 124.7 (+), 125.2 (+), 125.7 (+), 130.1 (q), 131.6 (q), 134.8 (q), 140.1 (q), 150.2 (q), 171.6 (q); HRMS (ESI): calcd for C18H23O2S (M + H)+ 303.1413; found 303.1418.Methyl 2-(5-([1,1′-biphenyl]-3-yl)-2-methylthiophen-3-yl)acetate (27)
Compound 27 was prepared from 22 (500 mg, 2.44 mmol) according to general procedure A. Purification by automated flash column chromatography (PE/EtOAc, 0–15% EtOAc) yielded 27 (569 mg, 72%) as yellow liquid. Rf: 0.50 (PE/EtOAc: 5/1); IR (neat) νmax: 1707, 1597, 1449, 1262, 1174, 755, 696; 1H-NMR (300 MHz, CDCl3): δ = 2.44 (s, 3H, thiophene-CH3), 3.58 (s, 2H, thiophene-CH2C(O)OCH3), 3.72 (s, 3H, thiophene-CH2C(O)OCH3), 7.19 (s, 1H, thiophene-H), 7.37–7.45 (m, 3H, Ph), 7.46–7.54 (m, 3H, Ph), 7.59–7.66 (m, 2H, Ph), 7.74–7.76 (m, 1H, Ph); 13C-NMR (75 MHz, CDCl3): δ = 2.44 (s, 3H, thiophene-CH3), 3.58 (s, 2H, thiophene-CH2C(O)OCH3), 3.72 (s, 3H, thiophene-CH2C(O)OCH3), 7.19 (s, 1H, thiophene-H), 7.37–7.42 (m, 1H, Ph), 7.43–7.47 (m, 3H, Ph), 7.48–7.54 (m, 2H, Ph), 7.60–7.65 (m, 2H, Ph), 7.74–7.76 (m, 1H, Ph); 13C-NMR (75 MHz, CDCl3): δ = 13.3 (+), 34.1 (−), 52.1 (+), 124.3 (+), 124.4 (+), 125.3 (+), 126.0 (+), 127.2 (+), 127.5 (+), 128.8 (+), 129.2 (+), 130.3 (q), 134.8 (q), 135.5 (q), 139.9 (q), 140.9 (q), 141.9 (q), 171.5 (q); HRMS (EI): calcd for C20H18O2S (M+˙) 322.1028; found 322.1032.Methyl 2-(5-(4-(tert-butyl)phenyl)-2-methylthiophen-3-yl)-2-oxoacetate (30)
Compound 30 was prepared from 28 (500 mg, 2.29 mmol) according to general procedure A. Purification by automated flash column chromatography (PE/EtOAc, 0–15% EtOAc) yielded 30 (350 mg, 48%) as dark yellow liquid. Rf: 0.67 (PE/EtOAc: 5/1); IR (neat) νmax: 2961, 2866, 1732, 1676, 1456, 1191, 1127, 993, 753; 1H-NMR (300 MHz, CDCl3): δ = 1.34 (s, 9H, tBu), 2.78 (s, 3H, thiophene-CH3), 3.96 (s, 3H, thiophene-CH2C(O)OCH3), 7.39–7.44 (m, 2H, Ph), 7.46–7.51 (m, 2H, Ph), 7.63 (s, 1H, thiophene-H); 13C-NMR (75 MHz, CDCl3): δ = 16.3 (+), 31.3 (+), 34.7 (q), 52.8 (+), 124.2 (+), 125.6 (+), 126.0 (+), 130.3 (q), 132.2 (q), 140.5 (q), 151.3 (q), 153.5 (q), 164.1 (q), 180.2 (q); HRMS (EI): calcd for C18H20O3S (M+˙) 316.1133; found 316.1139.Methyl 2-(5-([1,1′-biphenyl]-3-yl)-2-methylthiophen-3-yl)-2-oxoacetate (31)
Compound 31 was prepared from 28 (500 mg, 2.29 mmol) according to general procedure A. Purification by automated flash column chromatography (PE/EtOAc, 0–15% EtOAc) yielded 31 (537 mg, 70%) as yellow oil. Rf: 0.40 (PE/EtOAc: 5/1); IR (neat) νmax: 3028, 1724, 1668, 1598, 1464, 1197, 1132, 1000, 748, 695; 1H-NMR (300 MHz, CDCl3): δ = 2.81 (s, 3H, thiophene-CH3), 3.98 (s, 3H, thiophene-CH2C(O)OCH3), 7.36–7.43 (m, 1H, Ph), 7.45–7.49 (m, 2H, Ph), 7.50–7.57 (m, 3H, Ph), 7.60–7.65 (m, 2H, 4-H, Ph), 7.73–7.76 (m, 2H, Ph, thiophene-H); 13C-NMR (75 MHz, CDCl3): δ = 16.4 (+), 52.9 (+), 124.6 (+), 124.7 (+), 124.8 (+), 126.9 (+), 127.2 (+), 127.7 (+), 128.9 (+), 129.5 (+), 132.3 (q), 133.5 (q), 140.2 (q), 140.6 (q), 142.2 (q), 154.0 (q), 164.0 (q), 180.1 (q); HRMS (ESI): calcd for C20H15O2S (M + H+) − (H2O) 319.0787; found 319.0787.2-(5-(4-(tert-Butyl)phenyl)-2-methylthiophen-3-yl)-2-oxoacetamide (33)
Compound 33 was prepared from 30 (312 mg, 0.99 mmol) according to general procedure B. The amide 33 (250 mg, 84%) was obtained as light yellow solid and used without further purification. m.p.: 202 °C; IR (neat) νmax: 3395, 2955, 1712, 1651, 1454, 1191, 575; 1H-NMR (300 MHz, DMSO-d6): δ = 1.29 (s, 9H, tBu), 2.71 (s, 3H, thiophene-CH3), 7.36–7.48 (m, 2H, Ph), 7.51–7.59 (m, 2H, Ph), 7.74 (s, 1H, thiophene-H), 7.91 (s, 1H, NH), 8.26 (s, 1H, NH); 13C-NMR (75 MHz, DMSO-d6): δ = 15.4 (+), 30.9 (+), 34.3 (q), 124.4 (+), 125.0 (+), 126.0 (+), 129.7 (q), 133.0 (q), 138.9 (q), 150.5 (q), 150.6 (q), 167.0 (q), 185.5 (q); HRMS (ESI): calcd for C17H20NO2S (M + H+) 303.1240; found 303.1240.2-(5-([1,1′-Biphenyl]-3-yl)-2-methylthiophen-3-yl)-2-oxoacetamide (34)
Compound 34 was prepared from 31 (250 mg, 0.74 mmol) according to general procedure B. The amide 34 (224 mg, 94%) was obtained as light yellow solid and used without further purification. m.p.: 151 °C; IR (neat) νmax: 3393, 3302, 3184, 1721, 1652, 1597, 1456, 1352, 1196, 747, 689, 608; 1H-NMR (300 MHz, DMSO-d6): δ = 2.73 (s, 3H, thiophene-CH3), 7.39–7.44 (m, 1H, Ph), 7.47–7.56 (m, 3H, Ph), 7.58–7.66 (m, 2H, Ph), 7.71–7.75 (m, 2H, Ph), 7.83–7.85 (m, 1H, NH), 7.93 (bs, 2H, Ph, thiophene-H), 8.28 (bs, 1H, NH); 13C-NMR (75 MHz, DMSO-d6): δ = 15.4 (+), 123.4 (+), 124.4 (+), 125.5 (+), 126.4 (+), 126.8 (+), 127.7 (+), 128.9 (+), 129.9 (+), 133.1 (q), 133.2 (q), 138.7 (q), 139.5 (q), 141.1 (q), 151.1 (q), 166.9 (q), 185.6 (q); HRMS (ESI): calcd for C19H16NO2S (M + H+) 322.0896; found 322.0893.3-(5-(4-(tert-Butyl)phenyl)-2-methylthiophen-3-yl)-4-(5-chloro-2-methylthiophen-3-yl)-1H-pyrrole-2,5-dione (35)
Compound 35 was prepared from amide 23 (100 mg, 0.49 mmol) and ester 26 (178 mg, 0.59 mmol) according to general procedure C. Purification by automated flash column chromatography (PE/EtOAc, 0–25% EtOAc) yielded 35 (62 mg, 28%) as dark green solid. Rf: 0.47 (PE/EtOAc: 5/1); m.p.: 145 °C; IR (neat) νmax: 3055, 2961, 1707, 1459, 1338, 1181, 1018, 987, 825; 1H-NMR (300 MHz, DMSO-d6): δ = 1.28 (s, 9H, tBu), 1.88 (s, 3H, thiophene-CH3), 1.99 (s, 3H, thiophene-CH3), 7.02 (s, 1H, thiophene-H), 7.28 (s, 1H, thiophene-H), 7.40–7.49 (m, 4H, Ph), 11.27 (s, 1H, NH); 13C-NMR (75 MHz, DMSO-d6): δ = 14.0 (+), 14.2 (+), 30.9 (+), 34.2 (q), 124.0 (+), 124.3 (q), 124.7 (+), 125.9 (+), 126.7 (q), 127.8 (q), 127.9 (+), 130.2 (q), 132.7 (q), 134.1 (q), 139.3 (q), 139.5 (q), 139.8 (q), 150.3 (q), 171.0 (q), 171.1 (q); HRMS (ESI): calcd for C24H26ClN2O2S2 (M + NH4)+ 473.1119; found 473.1116.3-(5-([1,1′-Biphenyl]-3-yl)-2-methylthiophen-3-yl)-4-(5-chloro-2-methylthiophen-3-yl)-1H-pyrrole-2,5-dione (36)
Compound 36 was prepared from amide 23 (100 mg, 0.49 mmol) and ester 27 (160 mg, 0.49 mmol) according to general procedure C. Purification by automated flash column chromatography (PE/EtOAc, 0–25% EtOAc) yielded 36 (63 mg, 27%) as dark red solid. Rf: 0.16 (PE/EtOAc: 5/1); m.p.: 137 °C; IR (neat) νmax: 2736, 1705, 1338, 1022, 1006, 756, 700; 1H-NMR (300 MHz, DMSO-d6): δ = 1.91 (s, 3H, thiophene-CH3), 2.01 (s, 3H, thiophene-CH3), 7.03 (s, 1H, thiophene-H), 7.37–7.44 (m, 1H, Ph), 7.46–7.56 (m, 5H, thiophene-H, Ph), 7.58–7.63 (m, 1H, Ph), 7.69–7.75 (m, 2H, Ph), 7.77–7.79 (m, 1H, Ph), 11.30 (s, 1H, NH); 13C-NMR (75 MHz, DMSO-d6): δ = 14.1 (+), 14.2 (+), 123.2 (+), 124.1 (+), 124.3 (q), 125.1 (+), 126.1 (+), 126.6 (q), 126.7 (+), 127.7 (+), 127.9 (+), 128.0 (q), 128.9 (+), 129.8 (+), 132.8 (q), 133.6 (q), 134.0 (q), 139.4 (q), 139.5 (q), 139.6 (q), 140.2 (q), 141.1 (q), 171.0 (q), 171.1 (q); HRMS (ESI): calcd for C26H22ClN2O2S2 (M + NH4)+ 493.0806; found 493.0804.3-(5-([1,1′-Biphenyl]-3-yl)-2-methylthiophen-3-yl)-4-(5-(4-(tert-butyl)phenyl)-2-methylthiophen-3-yl)-1H-pyrrole-2,5-dione (37)
Compound 37 was prepared from amide 33 (260 mg, 0.86 mmol) and ester 27 (277 mg, 0.86 mmol) according to general procedure C. Purification by automated flash column chromatography (PE/EtOAc, 0–15% EtOAc) yielded 37 (155 mg, 33%) as purple solid. Rf: 0.13 (PE/EtOAc: 5/1); m.p.: 145 °C; IR (neat) νmax: 2921, 2851, 1707, 1334, 831, 756; 1H-NMR (300 MHz, DMSO-d6): δ = 1.27 (s, 9H, tBu), 2.02 (s, 6H, thiophene-CH3, thiophene-CH3), 7.31 (s, 1H, thiophene-H), 7.38–7.43 (m, 3H, Ph), 7.44–7.50 (m, 5H, thiophene-H, Ph), 7.50–7.52 (m, 1H, Ph), 7.54–7.56 (m, 1H, Ph), 7.58–7.61 (m, 1H, Ph), 7.68–7.71 (m, 2H, Ph), 7.76–7.78 (m, 1H, Ph), 11.27 (s, 1H, NH); 13C-NMR (75 MHz, DMSO-d6): δ = 14.3 (+), 31.0 (+), 34.3 (q), 123.2 (+), 124.1 (+), 124.2 (+), 124.8 (+), 125.4 (+), 126.0 (+), 126.1 (+), 126.8 (+), 127.7 (+), 128.2 (q), 128.3 (q), 128.9 (+), 129.9 (+), 130.3 (q), 133.7 (q), 133.9 (q), 134.0 (q), 139.4 (q), 139.5 (q), 139.6 (q), 139.8 (q), 140.1 (q), 141.1 (q), 150.3 (q), 171.6 (q), 171.7 (q); HR-MS (ESI): calcd for C36H32NO2S2 (M + H)+ 576.1879; found 576.1875.Acknowledgements
CF thanks the graduate research training group GRK 1910 for financial support.Notes and references
- J. Zhang, Q. Zou and H. Tian, Adv. Mater., 2013, 25, 378–399 CrossRef CAS PubMed.
- W. Szymański, J. M. Beierle, H. A. V. Kistemaker, W. A. Velema and B. L. Feringa, Chem. Rev., 2013, 113, 6114–6178 CrossRef PubMed.
- D. Gust, J. Andreasson, U. Pischel, T. A. Moore and A. L. Moore, Chem. Commun., 2012, 48, 1947–1957 RSC.
- S. Kawata and Y. Kawata, Chem. Rev., 2000, 100, 1777–1788 CrossRef CAS PubMed.
- C. C. Corredor, Z. L. Huang and K. D. Belfield, Adv. Mater., 2006, 18, 2910–2914 CrossRef CAS.
- V. A. Barachevsky, M. M. Krayushkin, V. V. Kyiko and E. P. Grebennikov, Phys. Status Solidi C, 2011, 8, 2841–2845 CrossRef CAS.
- D. Vomasta, C. Högner, N. R. Branda and B. König, Angew. Chem., Int. Ed., 2008, 47, 7644–7647 CrossRef CAS PubMed.
- D. Vomasta, A. Innocenti, B. König and C. T. Supuran, Bioorg. Med. Chem. Lett., 2009, 19, 1283–1286 CrossRef CAS PubMed.
- B. Reisinger, N. Kuzmanovic, P. Löffler, R. Merkl, B. König and R. Sterner, Angew. Chem., Int. Ed., 2014, 53, 595–598 CrossRef CAS PubMed.
- C. Falenczyk, M. Schiedel, B. Karaman, T. Rumpf, N. Kuzmanovic, M. Grøtli, W. Sippl, M. Jung and B. König, Chem. Sci., 2014, 5, 4794–4799 RSC.
- M. Singer and A. Jäschke, J. Am. Chem. Soc., 2010, 132, 8372–8377 CrossRef CAS PubMed.
- S. Barrois and H.-A. Wagenknecht, Beilstein J. Org. Chem., 2012, 8, 905–914 CrossRef CAS PubMed.
- H. Cahová and A. Jäschke, Angew. Chem., Int. Ed., 2013, 52, 3186–3190 CrossRef PubMed.
- M. R. Banghart, A. Mourot, D. L. Fortin, J. Z. Yao, R. H. Kramer and D. Trauner, Angew. Chem., Int. Ed., 2009, 48, 9097–9101 CrossRef CAS PubMed.
- T. Fehrentz, M. Schönberger and D. Trauner, Angew. Chem., Int. Ed., 2011, 50, 12156–12182 CrossRef CAS PubMed.
- I. Tochitsky, M. R. Banghart, A. Mourot, J. Z. Yao, B. Gaub, R. H. Kramer and D. Trauner, Nat. Chem., 2012, 4, 105–111 CrossRef CAS PubMed.
- A. Mourot, T. Fehrentz, Y. Le Feuvre, C. M. Smith, C. Herold, D. Dalkara, F. Nagy, D. Trauner and R. H. Kramer, Nat. Methods, 2012, 9, 396–402 CrossRef CAS PubMed.
- M. Schönberger and D. Trauner, Angew. Chem., Int. Ed., 2014, 53, 3264–3267 CrossRef PubMed.
- M. Schönberger, M. Althaus, M. Fronius, W. Clauss and D. Trauner, Nat. Chem., 2014, 6, 712–719 Search PubMed.
- I. Tochitsky, A. Polosukhina, V. E. Degtyar, N. Gallerani, C. M. Smith, A. Friedman, R. N. Van Gelder, D. Trauner, D. Kaufer and R. H. Kramer, Neuron, 2014, 81, 800–813 CrossRef CAS PubMed.
- W. A. Velema, J. P. van der Berg, M. J. Hansen, W. Szymanski, A. J. M. Driessen and B. L. Feringa, Nat. Chem., 2013, 5, 924–928 CrossRef CAS PubMed.
- O. Babii, S. Afonin, M. Berditsch, S. Reiβer, P. K. Mykhailiuk, V. S. Kubyshkin, T. Steinbrecher, A. S. Ulrich and I. V. Komarov, Angew. Chem., Int. Ed., 2014, 53, 3392–3395 CrossRef CAS PubMed.
- U. Al-Atar, R. Fernandes, B. Johnsen, D. Baillie and N. R. Branda, J. Am. Chem. Soc., 2009, 131, 15966–15967 CrossRef CAS PubMed.
- W. A. Velema, W. Szymanski and B. L. Feringa, J. Am. Chem. Soc., 2014, 136, 2178–2191 CrossRef CAS PubMed.
- H. M. D. Bandara and S. C. Burdette, Chem. Soc. Rev., 2012, 41, 1809–1825 RSC.
- G. Berkovic, V. Krongauz and V. Weiss, Chem. Rev., 2000, 100, 1741–1754 CrossRef CAS PubMed.
- Y. Yokoyama, Chem. Rev., 2000, 100, 1717–1740 CrossRef CAS PubMed.
- M. Irie, Chem. Rev., 2000, 100, 1685–1716 CrossRef CAS PubMed.
- H. Tian and S. Yang, Chem. Soc. Rev., 2004, 33, 85–97 RSC.
- P. Raster, S. Weiss, G. Hilt and B. König, Synthesis, 2011, 2011, 905–908 CrossRef.
- M. Dubernet, V. Caubert, J. Guillard and M.-C. Viaud-Massuard, Tetrahedron, 2005, 61, 4585–4593 CrossRef CAS.
- S. V. Shorunov, M. M. Krayushkin, F. M. Stoyanovich and M. Irie, Russ. J. Org. Chem., 2006, 42, 1490–1497 CrossRef CAS.
- A. El Yahyaoui, G. Félix, A. Heynderickx, C. Moustrou and A. Samat, Tetrahedron, 2007, 63, 9482–9487 CrossRef CAS.
- M. M. Krayushkin, V. Z. Shirinyan, L. I. Belen'kii, A. A. Shimkin, A. Y. Martynkin and B. M. Uzhinov, Russ. J. Org. Chem., 2002, 38, 1335–1338 CrossRef CAS.
- M. Ohsumi, T. Fukaminato and M. Irie, Chem. Commun., 2005, 3921–3923 RSC.
- M. T. Indelli, S. Carli, M. Ghirotti, C. Chiorboli, M. Ravaglia, M. Garavelli and F. Scandola, J. Am. Chem. Soc., 2008, 130, 7286–7299 CrossRef CAS PubMed.
- M. M. Faul, L. L. Winneroski and C. A. Krumrich, J. Org. Chem., 1998, 63, 6053–6058 CrossRef CAS PubMed.
- M. M. Faul, L. L. Winneroski and C. A. Krumrich, Tetrahedron Lett., 1999, 40, 1109–1112 CrossRef CAS.
- W. Steglich, Pure Appl. Chem., 1989, 81, 281–288 Search PubMed.
- T. Tamaoki and H. Nakano, Nat. Biotechnol., 1990, 8, 732–735 CrossRef CAS.
- P. D. Davis, C. H. Hill, G. Lawton, J. S. Nixon, S. E. Wilkinson, S. A. Hurst, E. Keech and S. E. Turner, J. Med. Chem., 1992, 35, 177–184 CrossRef CAS PubMed.
- D. A. E. Cross, A. A. Culbert, K. A. Chalmers, L. Facci, S. D. Skaper and A. D. Reith, J. Neurochem., 2001, 77, 94–102 CrossRef CAS PubMed.
- L. Meijer, M. Flajolet and P. Greengard, Trends Pharmacol. Sci., 2004, 25, 471–480 CrossRef CAS PubMed.
- E. Khalifa, J. H. Bieri and M. Viscontini, Helv. Chim. Acta, 1973, 56, 2911–2919 CrossRef CAS PubMed.
- J. T. van Herpt, M. C. A. Stuart, W. R. Browne and B. L. Feringa, Chem. Eng. J., 2014, 20, 3077–3083 CAS.
- K. Fujimoto, T. Maruyama, Y. Okada, T. Itou and M. Inouye, Tetrahedron, 2013, 69, 6170–6175 CrossRef CAS.
- K. Fuji, T. Kawabata and E. Fujita, Chem. Pharm. Bull., 1980, 28, 3662–3664 CrossRef CAS.
- J. W. Fisher and K. L. Trinkle, Tetrahedron Lett., 1994, 35, 2505–2508 CrossRef CAS.
- Y. Goldberg and H. Alper, J. Org. Chem., 1993, 58, 3072–3075 CrossRef CAS.
- O. E. Levy, E. L. Madison, J. E. Semple, A. P. Tamiz and M. Weinhouse, US Pat., WO0214349 (A2), 2002.
- C. Hamdouchi, US Pat., WO2013025424 (A1), 2013.
- A. McKillop, B. P. Swann and E. C. Taylor, J. Am. Chem. Soc., 1973, 95, 3340–3343 CrossRef CAS.
- T. Yamaguchi and M. Irie, Chem. Lett., 2004, 33, 1398–1399 CrossRef CAS.
- T. Yamaguchi, K. Uchida and M. Irie, J. Am. Chem. Soc., 1997, 119, 6066–6071 CrossRef CAS.
- M. Irie and K. Sayo, J. Phys. Chem., 1992, 96, 7671–7674 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional experimental data and 1H and 13C-NMR spectra of all prepared compounds. See DOI: 10.1039/c5ra00015g |
| This journal is © The Royal Society of Chemistry 2015 |