A molecular assembly of piperidine carboxylic acid dithiocarbamate on gold nanoparticles for the selective and sensitive detection of Al3+ ion in water samples†
Abstract
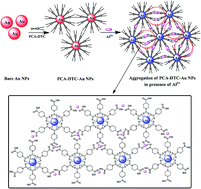
A sensitive and selective colorimetric method has been developed for the detection of Al3+ ions using 4-piperidine carboxylic acid dithiocarbamate-functionalized gold nanoparticles (PCA-DTC-Au NPs) as a probe. A high degree of PCA-DTC-Au NP aggregation was observed following the addition of Al3+ ion in the presence of 0.4 M NaCl in Tris–HCl buffer at pH 6.0. The Al3+ ions effectively induced the aggregation of PCA-DTC-Au NPs, resulting in a color change from red to blue. The characteristic surface plasmon resonance (SPR) peak of PCA-DTC-Au NPs was red-shifted to 660 nm. The Al3+ ion-induced aggregation of PCA-DTC-Au NPs was monitored by UV-visible spectrophotometry. The absorbance ratio (A660 nm/A523 nm) was linear for concentrations of Al3+ ion within the range 0.01–100 μM, with a correlation coefficient of R2 = 0.997. The probe was free from interference due to other metal ions and the color change was extremely specific towards Al3+ ion. The applicability of the present method in practical samples was studied by determining Al3+ ion in spiked water samples (drinking, tap, canal and river water) containing known concentrations of Al3+ ion.

 Please wait while we load your content...
Please wait while we load your content...