 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ozonolysis of a series of C7–C9 unsaturated biogenic aldehydes: reactivity study at atmospheric pressure†
Elizabeth Gaona
Colmán
a,
María B.
Blanco
*a,
Ian
Barnes
b and
Mariano A.
Teruel
*a
aINFIQC (CONICET, Facultad de Ciencias Químicas, Universidad Nacional de Córdoba) Dpto. de Fisicoquímica. Ciudad Universitaria, 5000 Córdoba, Argentina. E-mail: mteruel@fcq.unc.edu.ar; mblanco@fcq.unc.edu.ar; Fax: +54 351 4334188; Tel: +54 351 5353866 ext. 53531
bPhysikalische & Theoretische Chemie/FBC, Bergische Universitaet Wuppertal, Wuppertal, Germany
First published on 10th March 2015
Abstract
Rate coefficients for the reactions of O3 with trans-2-heptenal, trans-2-octenal and trans-2-nonenal have been determined at 298 K and (990 ± 10) mbar in an environmental chamber with in situ FTIR spectroscopy. The following rate coefficients in units of kO3 × 1018 (cm3 molecule−1 s−1) were obtained: (2.47 ± 0.73) for trans-2-heptenal, (2.37 ± 0.68) for trans-2-octenal and (2.05 ± 0.20) for trans-2-nonenal. It is shown that rate coefficients for the addition of O3 molecules and OH radicals to the double bond of alkenes and unsaturated and oxygenated volatile organic compounds (OVOCs) at 298 K are related to a good approximation by the expression: log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kOH = 0.16
kOH = 0.16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kO3 − 7.55. Furthermore, a correlation between the reactivity of unsaturated VOCs toward O3 molecules and the energies of the Highest Occupied Molecular Orbit (HOMO) of the unsaturated VOCs is presented and potential atmospheric implications of the results are discussed.
kO3 − 7.55. Furthermore, a correlation between the reactivity of unsaturated VOCs toward O3 molecules and the energies of the Highest Occupied Molecular Orbit (HOMO) of the unsaturated VOCs is presented and potential atmospheric implications of the results are discussed.
Introduction
Volatile Organic Compounds (VOCs) emitted from vegetation, often referred to as biogenic volatile organic compounds (BVOCs), are considered to be the main source of reactive species in the troposphere.1–3 Aldehydes play an important role in atmospheric chemistry since they are recognized as important key precursors in the formation of tropospheric ozone and OH radicals.4–7 They are also potential prolific sources of secondary organic aerosols through their reactions with atmospheric oxidants and photolysis.8Aldehydes are ubiquitous gaseous chemical constituents in the atmosphere and are emitted by a variety of sources. They arise, for example, from incomplete fossil fuel combustion, are emitted by vegetation and are produced during biomass burning. The photochemical oxidation of most VOCs in the atmosphere results in the formations of aldehydes to some degree.4–7
As mentioned above, in the troposphere aldehydes can be photolyzed6,7,9 and will also be subject to reaction with the main atmospheric oxidants OH radicals, NO3 radicals and O3 molecules. In the marine boundary layer and coastal regions reactions with Cl atoms can also be important. The combination of the photodissociation of aldehydes and reaction with the atmospheric oxidants represents, in many cases, an important source of free radicals in the lower atmosphere which can significantly influence the atmospheric oxidation capacity.7,10
A number of reviews on the gas-phase kinetics of the reactions of OH, NO3, O3 and Cl with different unsaturated aldehydes are available in the literature.4–7 For long chain (>C4) unsaturated aldehydes rate coefficients have been reported, by different groups using absolute and relative kinetic techniques, for the reaction of OH with E-2-pentenal, E-2-hexenal, E-2-heptenal, E-2-octenal and E-2-nonenal at room temperature and in some cases also as a function of temperature.6,7 In the case of NO3, rate coefficients have been reported at room temperature for the reaction of NO3 with five C5–C8 aldehydes, E-2-pentenal, E-2-hexenal, E-2-heptenal, Z-4-heptenal and E-2-octenal using different techniques. Again in some cases the reactions were also studied as a function of temperature.6,7 Rate coefficients for the reaction of Cl with C5–C7 2-enals (E-2-pentenal, E-2-hexenal, E-2-heptenal) have been reported in a single study by Rodríguez et al.11 who performed the measurements at 298 K using a relative kinetic method.
With regard to the ozonolysis of >C4 unsaturated aldehydes, which is the subject of this study, Sato et al.12 have determined rate coefficients at 298 K for the reaction of O3 with three pentenals, E-2-pentenal, 3-methyl-2-butenal and E-2-methyl-2-butenal, using the relative kinetic method. Grosjean et al.13 and Atkinson et al.14 have both measured the rate coefficient for the reaction of O3 with E-2-hexenal at room temperature using absolute and relative methods, respectively.
Since unsaturated aldehydes are released to the atmosphere in substantial amounts from combustion and vegetation and there are not many studies on the ozonolysis of these compounds, the motivation of this work was to investigate the ozonolysis of some longer carbon chain unsaturated aldehydes. The organic compounds studied in this work trans-2-heptenal, trans-2-octenal, trans-2-nonenal are biogenic aldehydes emitted from different types of vegetation.15–17 As part of a systematic study on the kinetics of the atmospheric reactions of different unsaturated oxygenated VOCs, we report in this study rate coefficients for the reactions of O3 molecules with the aforementioned aldehydes at 298 K and atmospheric pressure of synthetic air in a large volume photoreactor using the relative kinetic method:
CH3CH2CH2CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)H + O3 → Products, CHC(O)H + O3 → Products, | (1) |
CH3CH2CH2CH2CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)H + O3 → Products, CHC(O)H + O3 → Products, | (2) |
CH3CH2CH2CH2CH2CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)H + O3 → Products. CHC(O)H + O3 → Products. | (3) |
To the best of our knowledge rate coefficients of the above reactions have not been previously reported and this work, therefore, represents the first kinetic study of the reactions.
Additionally, the reactivity of the unsaturated aldehydes studied in this work together with other unsaturated VOCs toward O3 molecules has been correlated (i) with the reactivity of the same VOCs toward OH radicals and (ii) with the energies of the Highest Occupied Molecular Orbit (HOMO) of the unsaturated compounds. Tropospheric lifetimes for the studied aldehydes have been calculated and potential atmospheric implications assessed.
The kinetic data and correlations presented here help to improve our understanding of the atmospheric oxidation mechanisms of aldehydes. They also help in assessments of the potential contributions of the compounds to the oxidizing capacity of the tropospheric and photochemical smog and SOA formation.
Experimental section
All the experiments were performed in a 1080 L chamber at (298 ± 2) K in 990 ± 10 mbar of synthetic air. The chamber is composed of a cylindrical quartz vessel (total length 6.2 m and an inner diameter of 0.47 m) closed at both ends by aluminum end flanges. The metal flanges contain ports for the introduction of bath gases and reactants into the chamber. A magnetically coupled Teflon mixing fan is mounted inside the chamber to ensure homogeneous mixing of the reactants. The reactor can be evacuated by a pumping system consisting of a turbomolecular pump backed by a double stage rotary fore pump to 10−3 Torr. A White-type mirror system mounted internally in the chamber and coupled to a FTIR spectrometer Nicolet Nexus equipped with a liquid nitrogen cooled mercury–cadmium–telluride (MCT) detector enables ‘in situ’ monitoring of the reactants in the infrared range 4000–700 cm. The White mirror system was operated with the total optical absorption path length set to 484.7 m and infrared spectra were recorded with a spectral resolution of 1 cm−1, 100 interferograms were co-added per spectrum over a period of more than 1 min and 15 such spectra were recorded per experiment. The chamber is described in greater detail elsewhere.18,19 Ozone was added stepwise to mixtures containing the unsaturated aldehydes and reference compound. An electrical discharge in a flow of pure oxygen was used to generate ozone. The initial concentrations of reactants in (ppmV) were approximately: 0.9 for trans-2-heptenal; 0.8 for trans-2-octenal; 0.7 for trans-2-nonenal; 3.0 for ethene; 4.0 for 1,3-butadiene; 0.5 for vinyl propionate and 6–9 for ozone. The reactants were monitored at the following infrared frequencies (cm−1): trans-2-heptenal at 3077–2657; trans-2-octenal at 3000–2650; trans-2-nonenal at 3011–2649; ethene at 949; 1,3-butadiene at 908 and vinyl propionate at 1170. Typical IR spectra of the kinetics experiments performed for the reactions of O3 with trans-2-heptenal, trans-2-octenal and trans-2-nonenal are presented in the ESI as Fig. S1–S3,† respectively. In addition, the concentration–time profiles for the three unsaturated aldehydes (trans-2-heptenal, trans-2-octenal and trans-2-nonenal) are presented as Fig. S4–S6, respectively in the ESI.†Results and discussion
Rate coefficients for the reactions of the aldehydes with O3 were determined using the relative rate method in which the rates of decay of the aldehydes were monitored relative to the decay of reference compounds.| O3 + aldehyde → products, k1 | (4) |
| O3 + reference → products, k2 | (5) |
If the unsaturated aldehydes and reference organics are removed solely by reaction with O3 molecules according to eqn (4) and (5) then (6) is valid:
 | (6) |
 | ||
| Fig. 1 Plot of the kinetic data for the reaction of O3 molecules with trans-2-heptenal measured relative to ethene (□) and vinyl propionate (○) at (298 ± 2) K and atmospheric pressure of air. | ||
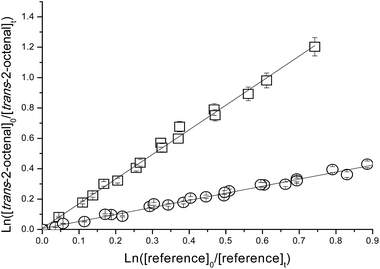 | ||
| Fig. 2 Plot of the kinetic data for the reaction of O3 molecules with trans-2-octenal measured relative to ethene (□) and vinyl propionate (○) at (298 ± 2) K and atmospheric pressure of air. | ||
 | ||
| Fig. 3 Plot of the kinetic data for the reaction of O3 molecules with trans-2-nonenal measured relative to ethene (□) and 1,3-butadiene (Δ) at (298 ± 2) K and atmospheric pressure of air. | ||
| Unsaturated aldehyde | Reference | k 1/k2 | k (O3+aldehyde) × 1018 (cm3 molecule−1 s−1) |
|---|---|---|---|
| trans-2-Heptenal + O3 | Ethene | 1.738 ± 0.048 | 2.38 ± 0.21 |
| Ethene | 1.745 ± 0.069 | 2.39 ± 0.23 | |
| Ethene | 1.878 ± 0.086 | 2.57 ± 0.27 | |
| Vinyl propionate | 0.484 ± 0.010 | 2.57 ± 0.68 | |
| Vinyl propionate | 0.456 ± 0.010 | 2.42 ± 0.65 | |
| Vinyl propionate | 0.479 ± 0.019 | 2.54 ± 0.73 | |
| Average | 2.47 ± 0.73 | ||
| trans-2-Octenal + O3 | Ethene | 1.626 ± 0.039 | 2.23 ± 0.19 |
| Ethene | 1.774 ± 0.064 | 2.43 ± 0.22 | |
| Ethene | 1.595 ± 0.023 | 2.19 ± 0.16 | |
| Vinyl propionate | 0.434 ± 0.010 | 2.30 ± 0.62 | |
| Vinyl propionate | 0.484 ± 0.010 | 2.57 ± 0.68 | |
| Vinyl propionate | 0.472 ± 0.010 | 2.50 ± 0.67 | |
| Average | 2.37 ± 0.68 | ||
| trans-2-Nonenal + O3 | 1,3-Butadiene | 0.322 ± 0.010 | 2.01 ± 0.19 |
| 1,3-Butadiene | 0.346 ± 0.010 | 2.16 ± 0.20 | |
| 1,3-Butadiene | 0.335 ± 0.010 | 2.09 ± 0.20 | |
| Ethene | 1.468 ± 0.041 | 2.01 ± 0.17 | |
| Ethene | 1.479 ± 0.037 | 2.03 ± 0.17 | |
| Ethene | 1.471 ± 0.016 | 2.02 ± 0.15 | |
| Average | 2.05 ± 0.20 | ||
The errors quoted for the rate coefficients for the reactions of O3 with the aldehydes in Table 1 are a combination of the 2σ statistical errors from the linear regression analyses of the plots plus the corresponding error of the reference reaction rate coefficient. As can be seen from Table 1 there is good agreement between the rate coefficients obtained for the reaction of O3 with the aldehydes using two different reference compounds. Because of this good agreement, we prefer to give final rate coefficients for the reactions which are an average of all the determinations, i.e.
| k(O3+trans-2-heptenal) = (2.47 ± 0.73) × 10−18 cm3 molecule−1 s−1 |
| k(O3+trans-2-octenal) = (2.37 ± 0.68) × 10−18 cm3 molecule−1 s−1 |
| k(O3+trans-2-nonenal) = (2.05 ± 0.20) × 10−18 cm3 molecule−1 s−1 |
It is well established that the ozonolysis of alkenes produces OH radicals which can interfere with relative kinetic studies of the type presented here. To minimize this inference an organic compound or CO is often added to the system to scavenge the OH radicals. The rate coefficients for the reactions of the unsaturated aldehydes under investigation in this study are very high, thus in order to effectively scavenge any OH radicals produced during the ozonolysis reaction high concentrations of a scavenger would be necessary. Since such high concentrations of the scavenger in the reaction system would render monitoring of the reactants in the infrared impossible, the experiments have been performed in the absence of a scavenger. We have obtained values of the rate coefficients for the reactions of O3 with the aldehydes using two reference compounds which are in excellent agreement with one another. Since the rate coefficients for the reactions of O3 with the reference compounds differ by a factor of approximately 4 and their rate coefficients with OH are all quite different we argue that any influence by OH in the reaction systems can not be very significant.
Since all of the hydrocarbons in the system will react with OH it would appear that in the systems investigated any interference by OH, that might possibly be occurring, is largely self-compensating. However, it is still important to keep in mind when making rate coefficient comparisons that the present values were determined in the absence of an OH radical scavenger.
The rate coefficients values obtained for the three reactions studied are very similar and within the error limits can be considered the same. The rate coefficients for the reactions of ozone with the three aldehydes have been estimated using the US EPA AOPWIN program which is based upon the structure–activity relationship (SAR) method described in Kwok and Atkinson.22 This estimation method predicts a rate coefficient value of 1.82 × 10−18 cm3 molecule−1 s−1 for all three of the unsaturated aldehydes which is lower than experimental values reported here by approximately 35% for trans-2-heptenal, 30% for trans-2-octenal and 13% for trans-2-nonenal. This agreement is quite acceptable considering the uncertainties in the rate coefficient determinations.
The rate coefficient of (2.0 ± 1.0) × 10−18 cm3 molecule−1 s−1 reported by Atkinson et al.14 for the reaction of O3 with trans-2-hexenal is very similar, within the error limits, to the rate coefficients determined in this work for the reactions of O3 with trans-2-heptenal, trans-2-octenal and trans-2-nonenal. This similarity in the reactivity for ≥ C6 unsaturated-2-enals toward O3 supports that above C6 further increases in the alkyl chain length of the aldehyde has a negligible effect on the magnitude of the electron donation to the double bond.
Rate coefficients for the addition of OH and NO3 radicals, Cl atoms and O3 molecules to alkenes and unsaturated VOCs have been shown previously to correlate with the energy of the HOMO of the different VOCs.23–26 The electron density in the π-bond that is attacked by the O3 molecule should be reflected in the energy of the highest occupied molecular orbital (EHOMO) with the lowest negative value being expected for the compound with the largest rate coefficient. To contribute to extend the correlations reported previously for the rate coefficients of OH and Cl reactions with EHOMO energies,27 a new correlation for the ozonolysis of different unsaturated VOCs has been determined using the Gaussian 03 package.28 The geometry optimizations and initial values of the energies were obtained at the B3LYP level with a 6-311G(d,p) bases set. The EHOMO calculated for the unsaturated aldehydes and the other unsaturated VOCs are listed in Table 2. Fig. 4 shows a plot of the natural logarithms of the O3 rate coefficients plotted as a function of the calculated EHOMO in eV. A linear relationship is obtained. The linear relationship in Fig. 4 is well described by:
−ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kO3 (cm3 molecule−1 s−1) = (9.52 ± 1.12)(−EHOMO) + (26.50 ± 2.35), r2 = 0.97 kO3 (cm3 molecule−1 s−1) = (9.52 ± 1.12)(−EHOMO) + (26.50 ± 2.35), r2 = 0.97 | (7) |
| Volatile organic compounds | k OH (cm3 molecule−1 s−1) | k O3 (cm3 molecule−1 s−1) | E homo (eV) | |
|---|---|---|---|---|
| a Ref. 33. b Ref.34. c This work. | ||||
| 1 | CH3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCHO, crotonaldehyde CHCHO, crotonaldehyde |
3.51 × 10−11a | 1.74 × 10−18a | −6.99986 |
| 2 | CH3CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCHO, trans-2-pentenal CHCHO, trans-2-pentenal |
2.35 × 10−11a | 1.59 × 10−18a | −6.97047 |
| 3 | CH3CH2CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCHO, trans-2-hexenal CHCHO, trans-2-hexenal |
2.95 × 10−11a | 2.0 × 10−18a | −6.96204 |
| 4 | H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)CHO, methacrolein C(CH3)CHO, methacrolein |
2.90 × 10−11a | 1.3 × 10−18a | −7.16639 |
| 5 | CH3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)CHO, trans-2-methyl-2-butenal C(CH3)CHO, trans-2-methyl-2-butenal |
4.08 × 10−11a | 5.34 × 10−18a | −6.96884 |
| 6 | CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCHO, Acrolein CHCHO, Acrolein |
1.99 × 10−11a | 2.61 × 10−18a | −7.22027 |
| 7 | CH3(CH2)3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCHO, trans-2-heptenal CHCHO, trans-2-heptenal |
2.45 × 10−11a | 2.47 × 10−18c | −6.95088 |
| 8 | CH3(CH2)4CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCHO, trans-2-octenal CHCHO, trans-2-octenal |
4.05 × 10−11b | 2.37 × 10−18c | −6.97129 |
| 9 | CH3(CH2)5CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCHO, trans-2-nonenal CHCHO, trans-2-nonenal |
4.35 × 10−11b | 2.05 × 10−18c | −6.96993 |
| 10 | CH3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, Propene CH2, Propene |
2.52 × 10−11a | 1.06 × 10−17a | −7.07197 |
| 11 | CH3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH3, trans-2-butene CHCH3, trans-2-butene |
7.07 × 10−11a | 2.38 × 10−16a | −6.64448 |
| 12 | CH3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH3, cis-2-butene CHCH3, cis-2-butene |
5.48 × 10−11a | 1.29 × 10−16a | −6.63849 |
| 13 | CH3CH2CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, 1-pentene CH2, 1-pentene |
2.74 × 10−11a | 9.97 × 10−18a | −7.05347 |
| 14 | C2H5CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH3, cis-2-pentene CHCH3, cis-2-pentene |
6.38 × 10−11a | 1.28 × 10−16a | −6.65101 |
| 15 | CH3CH2CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH3, cis-2-hexene CHCH3, cis-2-hexene |
6.19 × 10−11a | 1.06 × 10−16a | −6.64285 |
| 16 | CH3(CH2)5CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, 1-octene CH2, 1-octene |
3.62 × 10−11a | 1.01 × 10−17a | −7.02979 |
| 17 | C2H5CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH3, trans-2-pentene CHCH3, trans-2-pentene |
6.86 × 10−11a | 1.59 × 10−16a | — |
| 18 | CH3CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2CH3, cis-3-hexene CHCH2CH3, cis-3-hexene |
6.29 × 10−11a | 1.44 × 10−16a | — |
| 19 | CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)2, Isobutene C(CH3)2, Isobutene |
5.48 × 10−11a | 1.11 × 10−17a | — |
| 20 | CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)COOC2H5, ethyl methacrylate C(CH3)COOC2H5, ethyl methacrylate |
4.58 × 10−11a | 7.68 × 10−18a | — |
| 21 | CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)COOCH3, methyl methacrylate C(CH3)COOCH3, methyl methacrylate |
4.15 × 10−11a | 4.67 × 10−17a | — |
| 22 | CH3COOCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, vinyl acetate CH2, vinyl acetate |
2.49 × 10−11a | 3.2 × 10−18a | — |
| 23 | CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOO(CH2)3CH3, n-butyl acrylate CHCOO(CH2)3CH3, n-butyl acrylate |
2.28 × 10−11a | 2.4 × 10−18a | — |
| 24 | CH3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5, ethyl crotonate CHCOOC2H5, ethyl crotonate |
4.96 × 10−11 | 8.0 × 10−18a | — |
| 25 | CH3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH3, methyl crotonate CHCOOCH3, methyl crotonate |
4.65 × 10−11a | 4.4 × 10−18a | — |
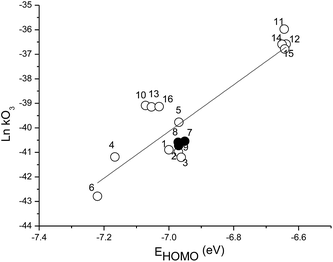 | ||
Fig. 4 Correlation of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kO3 (cm3 molecule−1 s−1) against EHOMO calculated using the Gaussian 3.0 package B3LYP/6-311G(d,p) for the reactions of volatile organic compounds with ozone. The numbers correspond to the compounds listed in Table 2 and the filled circles highlight the aldehydes, trans-2-heptenal, trans-2-octenal and trans-2-nonenal, studied in this work. kO3 (cm3 molecule−1 s−1) against EHOMO calculated using the Gaussian 3.0 package B3LYP/6-311G(d,p) for the reactions of volatile organic compounds with ozone. The numbers correspond to the compounds listed in Table 2 and the filled circles highlight the aldehydes, trans-2-heptenal, trans-2-octenal and trans-2-nonenal, studied in this work. | ||
The quality of the correlation is such that it can be used for reasonable estimations of rate coefficients for the reactions of O3 molecules with other unsaturated VOCs where data does not yet exist.
In addition, we present for the first time, a correlation between kOH and kO3 for a wide range of different unsaturated VOCs. The rate coefficients for the reactions of different unsaturated VOCs with O3 molecules and OH radicals at 298 K are listed in Table 2. The correlation obtained between the rate coefficients for the reactions of O3 molecules with a given alkene or unsaturated VOC and those for the corresponding reactions with OH radicals is shown in Fig. 5. A reasonable correlation is obtained and a least-squares treatment of the data points in Fig. 5 yields the following expression (with the rate coefficients in units of cm3 molecule−1 s−1):
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kOH = 0.16 kOH = 0.16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kO3 − 7.55, r2 = 0.95 kO3 − 7.55, r2 = 0.95 | (8) |
 | ||
Fig. 5 Linear free energy plot of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kOH against log kOH against log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kO3 at room temperature for a series of unsaturated volatile organic compounds. The numbers correspond to the compounds listed in Table 2 and the filled circles highlight the aldehydes, trans-2-heptenal, trans-2-octenal and trans-2-nonenal, studied in this work. kO3 at room temperature for a series of unsaturated volatile organic compounds. The numbers correspond to the compounds listed in Table 2 and the filled circles highlight the aldehydes, trans-2-heptenal, trans-2-octenal and trans-2-nonenal, studied in this work. | ||
The quality of this correlation is also such that it can be used to make reasonable estimations of rate coefficients for reactions of O3 molecules with other unsaturated VOCs where data does not yet exist.
The atmospheric lifetime of the studied aldehydes with respect to reaction with the main tropospheric oxidants can be estimated using the expression:
 | (9) |
| Aldehyde | k O3 cm3 molecule−1 s−1 | τ O3 (days) | k OH cm3 molecule−1 s−1 | τ OH (hours) | k NO3 cm3 molecule−1 s−1 | τ NO3 (hours) | k Cl cm3 molecule−1 s−1 | τ Cl (days) |
|---|---|---|---|---|---|---|---|---|
| a This work. b Ref. 35. c Ref. 34. d Ref. 36. e Ref. 37. f Ref. 11. | ||||||||
| trans-2-Heptenal | 2.47 × 10−18a | 7 | 2.45 × 10−11b | 6 | 0.23 × 10−13d | 0.4 | 2.40 × 10−10f | 5 |
| 5.3 × 10−15e | 2 | |||||||
| trans-2-Octenal | 2.37 × 10−18a | 7 | 40.5 × 10−12c | 3 | 5.6 × 10−15e | 2 | — | — |
| trans-2-Nonenal | 2.05 × 10−18a | 8 | 43.5 × 10−12c | 3 | — | — | — | — |
Degradation of longer chain aldehydes constitutes a significant source of aldehydes containing less carbon atoms than the precursor. In the presence of OH, NO3, O3 longer chain unsaturated aldehyde will probably be degraded to much shorter chain aldehydes, which have longer lifetimes and can contribute effectively to tropospheric ozone and SOA formation. In addition, longer chain aldehydes in the course of their degradation can undergo isomerization reactions forming carbonyl compounds which in further oxidation reactions can form thermally stable PAN type compounds.
For the reactions studied in this work we expect, based on existing ozonolysis studies, that pentanal, hexanal and heptanal will be major reaction products from the reactions of O3 with trans-2-heptenal, trans-2-octenal and trans-2-nonenal, respectively. Glyoxal will also be a major reaction product for all three aldehydes. However, the exact nature and yields of the products formed in the ozonolysis of the unsaturated aldehydes studied here still remains to be elucidated.
Acknowledgements
The authors wish to acknowledge MINCYT (Argentina)-PICT 2012-1740, CONICET(PIP)- GI 2010-2012-cod: 11220090100623 (Argentina), SECyT-UNC-14306/24. (Córdoba, Argentina) and the EU project EUROCHAMP-2 EU. E2-2013-02-27-0086 for financial support of this research. E.G.C. wishes to acknowledge to CONICET for a doctoral fellowship and support.References
- A. Guenther, C. N. Hewitt, D. Erickson, R. Fall, C. Geron, T. Graedel, P. Harley, L. Klinger, M. Lerdau, W. A. Mckay, T. Pierce, B. Scholes, R. Steinbrecher, R. Tallamraju, J.Taylor and P. Zimmerman, J. Geophys. Res.: Atmos., 1995, 100(D5), 8873–8892, DOI:10.1029/94JD02950.
- J. Kesselmeier and M. Staudt, J. Atmos. Chem., 1999, 33(1), 23–88, DOI:10.1023/a:1006127516791.
- J. E. Williams, P. F. J. Van Velthoven and C. A. M. Brenninkmeijer, Atmos. Chem. Phys., 2013, 13, 2857–2891, DOI:10.5194/acp-13-2857-2013.
- P. Carlier, H. Hannachi and G. Mouvier, Atmos. Environ., 1986, 20, 2079–2099, DOI:10.1016/0004-6981(86)90304-5.
- A. Mellouki, G. Le Bras and H. Sidebottom, Chem. Rev., 2003, 103(12), 5077–5096, DOI:10.1021/cr020526x.
- J. G. Calvert, A. Mellouki, J. J. Orlando, M. J. Pilling and T. J. Wallington, The Mechanisms of Atmospheric Oxidation of the Oxygenates, Oxford University Press, New York, 2011 Search PubMed.
- E. Jiménez and I. Barnes, Environment, Energy and Climate Change I: Environmental Chemistry of Pollutants and Wastes, in The Handbook of Environmental Chemistry, vol. 32, 2015 Search PubMed.
- A. W. H. Chan, M. N. Chan, J. D. Surratt, P. S. Chhabra, C. L. Loza, J. D. Crounse, L. D. Yee, R. C. Flagan, P. O. Wennberg and J. H. Seinfeld, Atmos. Chem. Phys., 2010, 10, 7169–7188 CAS.
- E. K. C. Lee and R. S. Lewis in Advances in photochemistry, ed. J. N. Pitts Jr, G. S. Hammond and K. Gollnick, vol. 12, Wiley, New York, 1980, pp. 1–96 Search PubMed.
- B. Finlayson- Pitts and J. Pitts Jr, Chemistry of the Upper and Lower Atmosphere-Theory, Experiments and Applications, ACADEMIC Press, 2000 Search PubMed.
- D. Rodríguez, A. Rodríguez, A. Notario, A. Aranda, Y. Díaz-de-Mera and E. Martínez, Atmos. Chem. Phys., 2005, 5, 3433–3440, DOI:10.5194/acp-5-3433-2005.
- K. Sato, B. Klotz, T. Taketsugu and T. Takayanagi, Phys. Chem. Chem. Phys., 2004, 6(15), 3969–3976, 10.1039/B402496F.
- E. Grosjean, D. Grosjean and J. H. Seinfeld, Int. J. Chem. Kinet., 1996, 28, 373–382 CrossRef CAS.
- R. Atkinson, J. Arey, S. M. Aschamann, S. B. Corchnoy and Y. Shu, Int. J. Chem. Kinet., 1995, 27, 941–955, DOI:10.1002/kin.550271002.
- K. Anjou and E. Von Sydow, Acta chemical scandinavica, 1967, 21, 945–952 CrossRef CAS.
- T. R. Kemp, Phytochemistry, 1975, 14, 2637–2638, DOI:10.1016/0031-9422(75)85240-x.
- Y. Xu and S. Barringer, J. Food Sci., 2010, 75, 268–273, DOI:10.1111/j.1750-3841.2010.01537.x.
- I. Barnes, K. H. Becker and T. Zhou, J. Atmos. Chem., 1993, 17, 353–373 CrossRef CAS.
- I. Barnes, K. H. Becker and N. Mihalopoulos, J. Atmos. Chem., 1994, 18, 267–289 CrossRef CAS.
- J. Treacy, M. E. Hag, D. O’Farrell and H. Sidebottom, Ber. Bunsenges. Phys. Chem., 1992, 96(3), 422–427 CrossRef CAS PubMed.
- W. M. Meylan and P. H. Howard, Chemosphere, 1993, 26, 2293–2299 CrossRef CAS.
- E. C. Kwok and R. Atkinson, Atmos. Environ., 1995, 29, 1685–1695 CrossRef CAS.
- E. Gaona Colmán, M. B. Blanco, I. Barnes and M. Teruel, Chem. Phys. Lett., 2013, 579, 11–15, DOI:10.1016/j.cplett.2013.05.049.
- C. Canosa-Mas, M. L. Flugge, M. King and R. Wayne, Phys. Chem. Chem. Phys., 2005, 7, 643–650, 10.1039/B416574H.
- M. D. King, C. E. Canosa-Mas and R. P. Wayne, Phys. Chem. Chem. Phys., 1999, 1, 2231–2238, 10.1039/a901193e.
- C. Pfrang, M. D. King, C. E. Canosa-Mas, M. Flugge and R. P. Wayne, Atmos. Environ., 2007, 41(8), 1792–1802, DOI:10.1016/j.atmosenv.2006.11.026.
- M. B. Blanco, I. Bejan, I. Barnes, P. Wiesen and M. A. Teruel, Atmos. Environ., 2009, 43(38), 5996–6002, DOI:10.1016/j.atmosenv.2009.08.032.
- M. J. Frisch, G. W. Schlegel, G. E. Scuseria, M. A. Robb, J. R. J. Cheeseman, A. Montgomery Jr, T. Vreven, K. N. Kudin, J. C. Burant and J. M. Millam, et al.Gaussian 03 Revision-D.01 Gaussian 03 Revision-D.01, Gaussian, Inc., Pittsburgh PA, 2003 Search PubMed.
- J. A. Logan, J. Geophys. Res.: Atmos., 1985, 90, 10463–10482, DOI:10.1029/JD090iD06p10463.
- R. Hein, P. J. Crutzen and M. Heimann, Global Biogeochem. Cycles, 1997, 11, 43 CrossRef CAS.
- O. W. Wingenter, M. K. Kubo, N. J. Blake, T. W. Smith, D. R. Blake and F. S. Rowland, J. Geophys. Res., 1996, 101, 4331–4340 CrossRef CAS.
- Y. Shu and R. Atkinson, J. Geophys. Res.: Atmos., 1995, 100(D4), 7275–7281, DOI:10.1029/95JD00368.
- NIST-Chemical Kinetics Database on the Web – Standard Reference Database17, Version 7.0 (Web Version), Release 1.6.3, Data Version, 2011 Search PubMed.
- T. Gao, J. M. Andino, C. C. Rivera and M. Francisco Márquez, Int. J. Chem. Kinet., 2009, 41, 483–489, DOI:10.1002/kin.20424.
- J. Albaladejo, B. Ballesteros, E. Jimenez, P. Martin and E. Martinez, Atmos. Environ., 2002, 36, 3231–3239, DOI:10.1016/S1352-2310(02)00323-0.
- Z. Zhao, S. Husainy and G. D. Smith, J. Phys. Chem. A, 2011, 115(44), 12161–12172, DOI:10.1021/jp206899w.
- J. Kerdouci, B. Picquet-Varrault, R. Durand-Jolibois, C. Gaimoz and J. F. J. Doussin, J. Phys. Chem. A, 2012, 116(41), 0135–10142, DOI:10.1021/jp3071234.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra17283c |
| This journal is © The Royal Society of Chemistry 2015 |
