4-Cyano-5-(2-thiophenyl)-pyrazoles are high affinity CB1 receptor ligands†
Stefano Altomonte*a,
Gemma L. Baillieb,
Ruth A. Rossb and
Matteo Zanda*ac
aKosterlitz Centre for Therapeutics, Institute of Medical Sciences, University of Aberdeen, Foresterhill, AB25 2ZD, Scotland, UK. E-mail: m.zanda@abdn.ac.uk
bMedical Sciences Building, University of Toronto, 1 King's College Circle, Toronto, Ontario, Canada M5S 1A8
cC.N.R.-Istituto di Chimica del Riconoscimento Molecolare, via Mancinelli 7, 20131 Milano, Italy
First published on 21st January 2015
Abstract
Pyrazoles bearing a 5-thiophenyl and a 4-cyano group were synthesised and tested for their affinity to the cannabinoid CB1 receptor showing in many cases single digit nanomolar Ki values and moderate to good selectivity towards the CB2 receptor. Some of these pyrazole ligands, such as 8g, displayed relatively low lipophilicity (experimental log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P < 4) and high calculated Topological Polar Surface Area (TPSA) (>90) suggesting that these compounds may behave as peripherally restricted CB1 ligands. Furthermore, 2-fluoroethyl carboxamides 8d, 8h and 8l are interesting candidates for further development into PET tracers.
P < 4) and high calculated Topological Polar Surface Area (TPSA) (>90) suggesting that these compounds may behave as peripherally restricted CB1 ligands. Furthermore, 2-fluoroethyl carboxamides 8d, 8h and 8l are interesting candidates for further development into PET tracers.
Introduction
Cannabinoid receptors (CBRs) belong to the family of G-protein coupled receptors (GPCRs). There are at least two classes of CBRs known: cannabinoid receptors subtype-1 (CB1), localised predominantly in the Central Nervous System (CNS) and cannabinoid receptors subtype-2 (CB2), mostly present peripherally in the immune system. The CB1 receptor has received considerable attention during the last 20 years due to its involvement in a number of disorders and pathologies, such as obesity, depression, schizophrenia and cancer. The rise and fall of the anti-obesity CNS-active CB1 inverse agonist Rimonabant (SR141716) suggested that peripherally restricted ligands of the CB1 receptor may be a better option, devoid of the severe side effects (mostly suicidal-tendencies and depression) of Rimonabant while maintaining the favourable biological and pharmacological properties of CB1 ligands.1 Therefore, a remarkable effort has been devoted to the design and development of CB1 ligands unable to penetrate the brain, and to the identification of the most important physico-chemical and structural properties responsible for reducing or suppressing the brain uptake of these molecules. Lipophilicity, number of hydrogen-bond donor functions and polar surface area (PSA) are among the most used metrics for estimating the capacity of a molecule that is not actively transported into the brain to undergo passive diffusion across the blood–brain–barrier (BBB). Various recent reviews have been dedicated to the important topic of peripherally-restricted CB1 ligands and to the different strategies pursed for achieving selective peripheral blockade of CB1 receptors.1–3In 2008, Tseng et al. reported CB1 receptor–ligand SAR studies on 5-thiophenyl pyrazoles carrying different aliphatic functionalities in position 5′ on the thiophene ring, such as either alkynyl or alkenyl chains, suggesting that lipophilic moieties in that position favourably interact with the binding site of the CB1 receptor.4 One of these analogues, TM38837, was shown to be a highly potent CB1 antagonist having very limited brain penetration.5
With the view of developing novel peripherally restricted CB1 ligands having reduced lipophilicity and increased PSA relative to TM38837 and its analogues, we decided to replace the 4-methyl/ethyl pyrazole substituent featured by these compounds with a cyano group, and investigate the effect of this structural change on CB1 and CB2 binding affinity and selectivity. Indeed, although the cyano group has been previously used as a pyrazole-substituent in a CB1 ligand,6 to the best of our knowledge neither the full scope of this strategy nor the concomitant replacement of the Rimonabant-type C-5 aryl group with a thiophenyl group have been reported yet. Furthermore, we decided to investigate the effect of incorporating a fluoroalkyl chain on 4-cyano-5-thiophenyl-pyrazoles with the view of producing, in future studies, a 18F-labelled cyano-pyrazole for PET imaging.
Results and discussion
Chemistry
The synthesis of 5-(5-bromo)thiophenyl-pyrazoles 5a–d and 5-(5-alkynyl)thiophenyl-pyrazoles 8a–l was performed according to an improved synthetic pathway previously described for the preparation of the 4-cyano pyrazole JHU75528.7 Compounds 5a–d and 8a–l were synthesised in 6 and 7 steps with 13–25% and 10–23% overall yields, respectively (Scheme 1 and 2). The hydrazone 1 was obtained in good yield by Japp–Klingemann reaction of an arenediazonium salt, prepared in situ from the commercially available 2,4-dichloro aniline, with ethyl 2-chloro-3-oxobutanoate. The condensation of the 2,4-dichlorophenyl hydrazone 1 with the 3-oxo-3-(2-thienyl)propionitrile to give the 4-cyano pyrazole 2 was performed employing triethyl amine as base in tert-butanol as solvent at 30 °C. Gao reported this combination was the best in order to limit by-products due to hydrolysis and to obtain the pyrazole ring in acceptable yields;7 however, since to the best of our knowledge, this was the first time that a keto-thiophene was employed as a nucleophile in this reaction, we decided to investigate whether different conditions could lead to higher yields (Table 1).| Entry | Base | Solvent | Temperature | Yielda |
|---|---|---|---|---|
| a Yields refer to the major product, isolated and purified by flash chromatography.b 18-crown-6 was employed to increase the availability of the “naked” nucleophile.c The hydrazone was added portionwise to the reaction mixture. | ||||
| 1 | NaOEt | EtOH (abs) | rt | 21 |
| 2 | tBuOK | THF (dry) | rt | 22 |
| 3 | LiHMDS | THF (dry) | −78 °C to rt | Decomposition |
| 4 | NaH | THF (dry) | 0 °C to rt | 39 |
| 5b | NaH | THF (dry) | rt | 36 |
| 6 | Et3N | tBuOH | 30 °C | 57 |
| 7c | Et3N | tBuOH | 30 °C | 73 |
The base LiHMDS in THF consumed rapidly almost all the β-cyano ketone giving a mixture of by-products (entry 3) whereas alkoxide bases, such as sodium ethoxide (entry 1) and potassium tert-butoxide (entry 2) gave the pyrazole 2 in poor yields. The yield slightly improved by using the inorganic base sodium hydride in THF (entry 4); it is worth noting that there was no significant difference when the reaction was carried out in the presence of a crown ether (entry 5). In agreement with the literature results,7 the use of triethyl amine in tert-butanol at 30 °C (entry 6) turned out to be the best choice; however, we noticed that the portion-wise addition of hydrazone 1 to the reaction mixture significantly increased the yield of 2 from 57 to 73% (entry 7).
The regioselective bromination of thiophene 2 at position 5 (Scheme 1) was accomplished using n-bromosuccinimide (NBS), in DMF. The reaction solvent was optimised as well and, although there were no significant differences in yields between ACN and DMF, the latter was preferred since the product 3 could be isolated by precipitation. It is worth noting that although relatively harsh conditions were employed in this step, no bromination of other positions on the thiophene ring was noticed. The bromo-thiophene 3 was then hydrolysed to the corresponding acid 4 with potassium hydroxide in methanol; to avoid the formation of by-products resulting from hydrolysis of the cyano group, the reaction was carried out at room temperature. The carboxylic acid 4 was then converted into the corresponding acyl chloride by thionyl chloride in toluene and then coupled to different amines to afford the amides 5a–d (see Table 2 for the yields).
Alternatively (Scheme 2), bromo-thiophene 3 was coupled with three different alkynes via a Sonogashira reaction to afford the 5-alkynyl-pyrazoles 6a–c in very good yields (88–90%). Since the starting alkynes were volatile, all the reactions were carried out in sealed vessels. Compounds 6a–c were hydrolysed to the corresponding acids 7a–c and then coupled to several amines, via the corresponding acyl chlorides, affording the amides 8a–l (Table 2). It is worth noting that it was possible to perform the syntheses without any chromatographic purification of 5a–d until the final acylation of the amines and of 8a–l until the Sonogashira coupling step.
Binding affinity and SAR
Binding affinity tests for the cannabinoid receptors were performed on all compounds 5 and 8 by means of radio-receptor binding assays using the protocol previously described.8 The results are summarised in Table 3.| Compound | CB1 Ki (nM) | CB2 Ki (nM) | Ki CB2/Ki CB1 | Experimental log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pa Pa |
|---|---|---|---|---|
a Determined experimentally by means of RP-HPLC (see Experimental section for details).b TPSA13 (Å2) = 73.95.c TPSA13 (Å2) = 100.25.d TPSA13 (Å2) = 108.09.e TPSA13 (Å2) = 70.71.f TPSA13 (Å2) = 50.16.g log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D 7.4 value reported by the authors.14 D 7.4 value reported by the authors.14 |
||||
| 5ab | 13.7 ± 4.5 | 2307.0 ± 528.7 | 177 | 4.0 ± 0.2 |
| 5bc | 30.3 ± 8.2 | 628.8 ± 252.1 | 20 | 4.4 ± 0.2 |
| 5cd | 296.1 ± 99.9 | 5641.0 ± 2600.0 | 19 | 3.3 ± 0.2 |
| 5de | 304.0 ± 104.5 | 1245.0 ± 532.3 | 4 | 3.6 ± 0.2 |
| 8ab | 1.0 ± 0.3 | 59.3 ± 18.7 | 59 | 4.8 ± 0.2 |
| 8bc | 6.5 ± 1.2 | 206.7 ± 61.5 | 32 | 5.2 ± 0.3 |
| 8cd | 5.3 ± 2.2 | 97.0 ± 38.1 | 18 | 4.0 ± 0.2 |
| 8de | 5.9 ± 2.1 | 156.0 ± 30.2 | 26 | 4.4 ± 0.2 |
| 8eb | 1.8 ± 0.8 | 96.8 ± 18.4 | 53 | 4.5 ± 0.2 |
| 8fc | 5.4 ± 1.6 | 102.8 ± 32.2 | 20 | 4.9 ± 0.3 |
| 8gd | 11.4 ± 3.5 | 157.1 ± 48.7 | 14 | 3.7 ± 0.2 |
| 8he | 13.0 ± 4.5 | 550.6 ± 197.1 | 42 | 4.1 ± 0.2 |
| 8ib | 1.1 ± 0.2 | 36.1 ± 10.8 | 36 | 5.3 ± 0.3 |
| 8jc | 1.3 ± 0.4 | 34.4 ± 12.5 | 26 | 5.7 ± 0.3 |
| 8kd | 5.3 ± 1.6 | 55.7 ± 13.5 | 10 | 4.5 ± 0.2 |
| 8le | 10.3 ± 3.2 | 104.5 ± 30.2 | 10 | 4.8 ± 0.2 |
| SR141716f | 2.2 | 4900 | 2227 | 4.7 ± 0.2 |
| NESS098Af | 17.4 (ref. 12) | 781 (ref. 12) | 45 | n.d. |
| JHU75528 | 11 ± 7 | n.d. | n.d. | 3.3g |
| O-1398 | 852 ± 175 | n.d. | n.d. | n.d. |
The cyano pyrazole 5a is an analogue of NESS098A (Fig. 1) and is structurally related to the inverse agonist SR141716 (Rimonabant, Fig. 1). Replacement of the methyl in position 4 on the pyrazole ring with the more polar cyano group increased 4-fold the CB1/CB2 selectivity of 5a relative to NESS098A (Table 3), while maintaining a similar affinity. Furthermore, 5a showed a reduced lipophilicity (experimental log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) relative to that displayed by Rimonabant. Slightly lower CB1 affinity and significantly lower CB1/CB2 selectivity were observed for 5b, having R1 = N-Boc-4-piperidyl as carboxamide substituent. A significant drop of CB1 affinity was measured for compounds 5c,d, having respectively a dioxo-thiomorpholine and 2-fluoroethyl carboxamide residues as R1. The introduction of alkynyl substituents on the 5-thiophenyl ring in compounds 8 brought about a significant general improvement of the CB1 affinity relative to the corresponding precursors 5a–d, as demonstrated by the fact that all compounds 8a–l have CB1 Ki in the 1.0–13.0 nM range. On the other hand, the nature of this alkynyl residue R2 had little effect on CB1 affinity and CB1/CB2 selectivity. In contrast, the nature of the carboxamido residue R1 had a more profound effect on both CB1 affinity and CB1/CB2 selectivity. In fact all the compounds having a Rimonabant-type R1 = piperidyl (8a, 8e, 8i) showed higher CB1 affinity (Ki ca. 1 nM) and CB1/CB2 selectivity than their analogues having the same alkynyl R2 and different carboxamides R1 within the three series 8a–d, 8e–h and 8i–l. It is worth noting that 2-fluoroethyl carboxamides 8d, 8h and 8l, which should be considered candidate PET tracers as the fluorine atom is potentially amenable to 18F-radiofluorination, all showed Ki CB1 ca. 10 nM, which is essentially equal to that of the CB1 PET tracer JHU75528. Furthermore 8h displayed a good CB1/CB2 selectivity = 42, combined with a significantly lower experimental log
P) relative to that displayed by Rimonabant. Slightly lower CB1 affinity and significantly lower CB1/CB2 selectivity were observed for 5b, having R1 = N-Boc-4-piperidyl as carboxamide substituent. A significant drop of CB1 affinity was measured for compounds 5c,d, having respectively a dioxo-thiomorpholine and 2-fluoroethyl carboxamide residues as R1. The introduction of alkynyl substituents on the 5-thiophenyl ring in compounds 8 brought about a significant general improvement of the CB1 affinity relative to the corresponding precursors 5a–d, as demonstrated by the fact that all compounds 8a–l have CB1 Ki in the 1.0–13.0 nM range. On the other hand, the nature of this alkynyl residue R2 had little effect on CB1 affinity and CB1/CB2 selectivity. In contrast, the nature of the carboxamido residue R1 had a more profound effect on both CB1 affinity and CB1/CB2 selectivity. In fact all the compounds having a Rimonabant-type R1 = piperidyl (8a, 8e, 8i) showed higher CB1 affinity (Ki ca. 1 nM) and CB1/CB2 selectivity than their analogues having the same alkynyl R2 and different carboxamides R1 within the three series 8a–d, 8e–h and 8i–l. It is worth noting that 2-fluoroethyl carboxamides 8d, 8h and 8l, which should be considered candidate PET tracers as the fluorine atom is potentially amenable to 18F-radiofluorination, all showed Ki CB1 ca. 10 nM, which is essentially equal to that of the CB1 PET tracer JHU75528. Furthermore 8h displayed a good CB1/CB2 selectivity = 42, combined with a significantly lower experimental log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P than that of Rimonabant. All together, the data above suggest that 8h is an interesting candidate for further development into an experimental PET tracer for CB1 imaging. It is also noteworthy that the optimised fluoroethylamide compounds 8h,d,l displayed much higher CB1 affinity than that reported for the Rimonabant-type fluoroethylamide analogue O-1398 (Fig. 1 and Table 3),9 and even the bromothiophenyl precursor 5d showed 4-fold higher CB1 activity than O-1398. Considering the nature of the CB receptors, which are transmembrane receptors and generally prefer ligands with high lipophilicity,10 it is not surprising that compound 5c, which has the lowest lipophilicity (log
P than that of Rimonabant. All together, the data above suggest that 8h is an interesting candidate for further development into an experimental PET tracer for CB1 imaging. It is also noteworthy that the optimised fluoroethylamide compounds 8h,d,l displayed much higher CB1 affinity than that reported for the Rimonabant-type fluoroethylamide analogue O-1398 (Fig. 1 and Table 3),9 and even the bromothiophenyl precursor 5d showed 4-fold higher CB1 activity than O-1398. Considering the nature of the CB receptors, which are transmembrane receptors and generally prefer ligands with high lipophilicity,10 it is not surprising that compound 5c, which has the lowest lipophilicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 3.3 ± 0.2) displayed one of the lowest CB1 affinities whereas, for instance, the N-BOC protected 8j with its high log
P = 3.3 ± 0.2) displayed one of the lowest CB1 affinities whereas, for instance, the N-BOC protected 8j with its high log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P value of 5.7 ± 0.3 showed one of the best CB1 affinities. Finally, as shown in Tables 1 and 2, compounds having R1 = N-Boc-4-piperidyl (5b, 8b,f,j) or dioxo-thiomorpholine (5c, 8c,g,k) display calculated Topological Polar Surface Area (TPSA) values11 >90, which is the generally accepted cut-off value for brain penetration,11 whereas Rimonabant has a TPSA ∼50, so these compounds might be peripherally restricted.
P value of 5.7 ± 0.3 showed one of the best CB1 affinities. Finally, as shown in Tables 1 and 2, compounds having R1 = N-Boc-4-piperidyl (5b, 8b,f,j) or dioxo-thiomorpholine (5c, 8c,g,k) display calculated Topological Polar Surface Area (TPSA) values11 >90, which is the generally accepted cut-off value for brain penetration,11 whereas Rimonabant has a TPSA ∼50, so these compounds might be peripherally restricted.
Functional assays
Two of the novel ligands, 8d and 8h which are the most suitable for prospective PET imaging applications, were investigated using a functional assay, the [35S]GTPγS binding assay (for details see ESI†). 8d caused a significant increase in [35S]GTPγS binding to 42.1% (95% confidence limits, 30 – 55) with an EC50 value of 255 nM (95% confidence limits, 28 – 2351) when investigated alone. When 100 nM was used to antagonize the CB1 receptor agonist CP55940 there was an increase in the EC50 value from 21.3 nM (95% confidence limits, 4 – 104) in the presence of vehicle, to 313 nM (95% confidence limits, 18 – 5536) in the presence of 100 nM 8d.8h also caused a significant increase in [35S]GTPγS binding to 44.7% (95% confidence limits, 40 – 58) with an EC50 value of 123 nM (95% confidence limits, 13 – 1131) when investigated alone. When 100 nM was used to antagonize the CB1 receptor agonist CP55940 there was an increase in the EC50 value from 3.7 nM (95% confidence limits, 0.3 – 43) in the presence of vehicle, to 268 nM (95% confidence limits, 62 – 1159) in the presence of 100 nM 8h.
These data show that both the tested compounds showed a significant increase in the EC50 values and should be therefore considered antagonists of CP55940 for the CB1 receptor. Given the structural similarity of 8d,h with all of the other novel CB1 ligands 5 and 8 we confidently assume that all of these compounds are also CB1 antagonists.
Conclusion
Replacement of the Rimonabant-type C-5 aryl ring with a thiophenyl bioisostere and of the C-3 methyl group with a more polar cyano group generated a novel class of pyrazolyl cannabinoid receptor subtype 1 ligands, that were synthesised in 6 (5a–d) and 7 steps (8a–l) with 13–25% and 10–23% overall yields, respectively. Among the 5-bromothiophenyl derivatives 5a–d, only 5a displayed high CB1 affinity and CB1/CB2 selectivity and modification of the 1-piperidyl carboxamide residue R1 caused a decrease of both affinity and selectivity. Replacement of the bromine atom with an alkynyl residue as in compounds 8a–l resulted in a generally increased CB1 affinity (1.0–13.0 nM) and maintained good CB1/CB2 selectivity (ratios in the range 10 to 59). 2-Fluoroethyl carboxamides 8d, 8h and 8l are interesting candidates for further development into PET tracers, particularly 8h that showed CB1 Ki = 13.0 nM and CB1/CB2 selectivity = 42. Both lipophilicity and TPSA could be efficiently tuned in these class of CB1 ligands. In fact, compound 8g showed low lipophilicity (experimental log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 3.7) relative to most known CB1 ligands, combined with a good affinity (CB1 Ki = 11.4 nM), whereas 8j had very high lipophilicity (log
P = 3.7) relative to most known CB1 ligands, combined with a good affinity (CB1 Ki = 11.4 nM), whereas 8j had very high lipophilicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 5.7), very high affinity (CB1 Ki = 1.1 nM) and good selectivity (CB1/CB2 = 36). However, the same compound 8j and other analogues (8b,c,f,g,k) feature high calculated TPSA (>100) so they may behave as peripherally restricted CB1 ligands. In particular, the pentynyl thiophene 8c showed similar biological and physico-chemical properties relative to the well-studied peripherally restricted cannabinoid ligand AM6545.15 Functional binding assays performed on two of the novel ligands, 8d and 8h, showed that these compounds are competitive antagonists of CP55940 for the CB1 receptor.
P = 5.7), very high affinity (CB1 Ki = 1.1 nM) and good selectivity (CB1/CB2 = 36). However, the same compound 8j and other analogues (8b,c,f,g,k) feature high calculated TPSA (>100) so they may behave as peripherally restricted CB1 ligands. In particular, the pentynyl thiophene 8c showed similar biological and physico-chemical properties relative to the well-studied peripherally restricted cannabinoid ligand AM6545.15 Functional binding assays performed on two of the novel ligands, 8d and 8h, showed that these compounds are competitive antagonists of CP55940 for the CB1 receptor.
Experimental section
Chemistry
NMR data were recorded on Bruker ADVANCE III for 1H at 400.13 MHz, for 13C at 100.58 MHz and for 19F at 376.45 MHz. 1H NMR chemical shifts are reported relative to TMS, and the solvent resonance was employed as the internal standard (CDCl3 δ = 7.26). 13C NMR spectra were recorded with complete proton decoupling, and the chemical shifts are reported relative to TMS with the solvent resonance as internal standard (CDCl3 δ = 77.0). 19F NMR spectra were referenced to CFCl3 as the external standard. All chemical shift (σ) are reported in parts per million (ppm) downfield from TMS and coupling constant (J) in Hertz. Splitting patterns are reported as follows: s, singlet; d, doublet; dd, doublet of doublets; ddd, doublet of doublet of doublets; t, triplet; td, triplet of doublets; appt, apparent triplet; q, quadruplet; qd, quadruplet of doublets; m, multiplet; br, broad signal.
Mass Analysis was performed using an Agilent 1200 HPLC system coupled to an Agilent G6120 single quadrupole detector equipped with Electrospray ionization (ESI) source in direct infusion modality.
Lipophilicities were determined using a Reverse Phase (RP)-HPLC with an Agilent 1200 HPLC system equipped with a DAD, analytical Phenomenex Luna C-18 column (250 × 4.60 mm l × ID, particle size: 5 μ) and an ESI-MS detector.
HRMS analysis were performed by the EPSRC National Mass Spectrometry Service Centre (Swansea, UK).
All reactions were carried out in oven- or flame-dried glassware under nitrogen atmosphere, unless stated otherwise, and were magnetically stirred and monitored by TLC on silica gel (60 F254 pre-coated glass plates, 0.25 mm thickness).
Visualization was accomplished using irradiation with a UV lamp (λ = 254 nm or λ = 365 nm), and/or staining with potassium permanganate or ceric ammonium molybdate solution.
Purification of reaction products was performed using flash chromatography on silica gel (60 Å, particle size 40–63 μm) according to the procedure of Still and co-workers.16
Yields refer to chromatographically and spectroscopically pure compounds, unless stated otherwise.
Ethyl 2-chloro-2-(2-(2,4-dichlorophenyl)hydrazono)acetate (1). 2,4-Dichloroaniline (5.00 g, 30.56 mmol) was stirred at room temperature in an aqueous solution of HCl (7% v/v, 187 ml) until complete dissolution. The reaction mixture was cooled to 0 °C with ice and a solution of sodium nitrite (2.22 g, 31.17 mmol) in water (15 ml) was added dropwise for 1 hour. This cold orange mixture was cannulated into a cold solution of sodium acetate (2.43 g, 29.33 mmol) and ethyl 2-chloro-3-oxobutanoate (5.29 g, 30.56 mmol) in ethanol (305 ml). The temperature was allowed to reach the room temperature and the reaction was stirred for 2 h. The yellow precipitate formed was filtered, washed with water and dried overnight in a desiccator containing P2O5 to give the desired solid product 1 (84%). 1H NMR (400 MHz, chloroform-d) δ 8.74 (s, 1H), 7.56 (d, J = 8.8 Hz, 1H), 7.36 (d, J = 2.3 Hz, 1H), 7.26 (ddd, J = 8.8, 2.3, 0.6 Hz, 1H), 4.40 (q, J = 7.1 Hz, 2H), 1.41 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 159.2, 136.6, 129.0, 128.4, 127.7, 119.4, 119.2, 116.6, 63.1, 14.2. ESMS, calculated m/z C10H9Cl3N2O2 295 [M + H]+, found m/z (relative intensity) 317 [M + Na]+ (95).
Ethyl 4-cyano-1-(2,4-dichlorophenyl)-5-(thiophen-2-yl)-1H-pyrazole-3-carboxylate (2). A solution of 3-oxo-3-(2-thienyl)propionitrile (0.95 g, 6.11 mmol) and Et3N (3.1 ml, 22.23 mmol) in tert-butanol (15 ml) was magnetically stirred at 30 °C for 30 min. To this reaction mixture, compound 1 (2.00 g, 5.56 mmol) was added portionwise in 3 h. The reaction was kept at 30 °C and stirred for 16 h. The brown precipitate was filtered, dissolved in DCM and washed with water (3 × 20 ml). The organic phase was dried over anhydrous Na2SO4 and evaporated. The crude product was purified by column chromatography on silica gel (Hex/EtOAc 70
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) to afford the product 2 as a pale yellow solid (73%). 1H NMR (400 MHz, chloroform-d) δ 7.62 (dd, J = 3.8, 1.2 Hz, 1H), 7.56 (dd, J = 1.8, 0.7 Hz, 1H), 7.49–7.41 (m, 3H), 7.11 (dd, J = 5.1, 3.8 Hz, 1H), 4.52 (q, J = 7.1 Hz, 2H), 1.47 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 159.5, 145.9, 145.7, 138.1, 134.3, 134.0, 131.0, 130.7, 130.6, 130.1, 128.5, 128.0, 125.4, 112.7, 93.3, 62.4, 14.2. ESMS, calculated m/z C17H11Cl2N3O2S 392 [M + H]+, found m/z (relative intensity) 392.0 [M + H]+ (100).
30) to afford the product 2 as a pale yellow solid (73%). 1H NMR (400 MHz, chloroform-d) δ 7.62 (dd, J = 3.8, 1.2 Hz, 1H), 7.56 (dd, J = 1.8, 0.7 Hz, 1H), 7.49–7.41 (m, 3H), 7.11 (dd, J = 5.1, 3.8 Hz, 1H), 4.52 (q, J = 7.1 Hz, 2H), 1.47 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 159.5, 145.9, 145.7, 138.1, 134.3, 134.0, 131.0, 130.7, 130.6, 130.1, 128.5, 128.0, 125.4, 112.7, 93.3, 62.4, 14.2. ESMS, calculated m/z C17H11Cl2N3O2S 392 [M + H]+, found m/z (relative intensity) 392.0 [M + H]+ (100).
Ethyl 5-(5-bromothiophen-2-yl)-4-cyano-1-(2,4-dichlorophenyl)-1H-pyrazole-3-carboxylate (3). N-Bromosuccinimide (4.13 g, 22.95 mmol) was added portionwise to a magnetically stirred solution of 2 (3.33 g, 7.65 mmol) in DMF (26 ml), cooled with ice. The reaction was heated to 80 °C and stirred for 16 h. The reaction was quenched with a saturated solution of sodium thiosulphate. The precipitate was filtered and dissolved in a small portion of DCM. The organic phase was washed with water, dried over anhydrous Na2SO4 and concentrate under reduced pressure. The residue was purified by column chromatography on silica gel (Hex/EtOAc 80
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) to give the desired product 3 as a pale yellow solid (79%). 1H NMR (400 MHz, chloroform-d) δ 7.59 (dd, J = 1.9, 0.7 Hz, 1H), 7.47–7.45 (m, 2H), 7.40 (d, J = 4.1 Hz, 1H), 7.07 (d, J = 4.1 Hz, 1H), 4.52 (q, J = 7.1 Hz, 2H), 1.46 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 159.3, 145.9, 144.6, 138.4, 133.9, 133.9, 131.0, 130.9, 130.9, 130.8, 128.7, 126.7, 118.2, 112.4, 93.3, 62.5, 14.2. ESMS, calculated m/z C17H10BrCl2N3O2S 470 [M + H]+, found m/z (relative intensity) 469.9 [M + H]+ (29).
20) to give the desired product 3 as a pale yellow solid (79%). 1H NMR (400 MHz, chloroform-d) δ 7.59 (dd, J = 1.9, 0.7 Hz, 1H), 7.47–7.45 (m, 2H), 7.40 (d, J = 4.1 Hz, 1H), 7.07 (d, J = 4.1 Hz, 1H), 4.52 (q, J = 7.1 Hz, 2H), 1.46 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 159.3, 145.9, 144.6, 138.4, 133.9, 133.9, 131.0, 130.9, 130.9, 130.8, 128.7, 126.7, 118.2, 112.4, 93.3, 62.5, 14.2. ESMS, calculated m/z C17H10BrCl2N3O2S 470 [M + H]+, found m/z (relative intensity) 469.9 [M + H]+ (29).
5-(5-Bromothiophen-2-yl)-4-cyano-1-(2,4-dichlorophenyl)-1H-pyrazole-3-carboxylic acid (4). To a solution of bromo ester 3 (0.10 g, 0.20 mmol) in methanol (4 ml) was added a solution of potassium hydroxide (0.8 g, 1.2 mmol) in methanol (3 ml) dropwise at room temperature. The reaction mixture was stirred for 3 h. After the hydrolysis was complete, the reaction mixture was poured into ice-water and acidified with 2N hydrochloric acid. The precipitate was filtered, washed with water, and dried under vacuum to give the acid 4 (87%) as a white solid. 1H NMR (400 MHz, methanol-d4) δ 7.82 (d, J = 2.2 Hz, 1H), 7.74 (d, J = 8.5 Hz, 1H), 7.64 (dd, J = 8.5, 2.2 Hz, 1H), 7.36 (d, J = 4.1 Hz, 1H), 7.21 (d, J = 4.1 Hz, 1H); 13C NMR (101 MHz, MeOD) δ 160.7, 146.3, 144.6, 138.2, 134.1, 133.4, 131.3, 131.2, 130.9, 130.3, 128.8, 126.8, 117.6, 112.1, 93.1. ESMS, calculated m/z C15H6BrCl2N3O2S 442 [M + H]+, found m/z (relative intensity) 441.8 [M + H]+ (26).
5-(5-Bromothiophen-2-yl)-4-cyano-1-(2,4-dichlorophenyl)-1H-pyrazole-3-carbonyl chloride. A solution of the acid 4 (0.63 g, 1.39 mmol) and thionyl chloride (306 μl, 4.18 mmol) in toluene (9 ml) was refluxed for 3 h. Solvent was evaporated under reduced pressure and the residue was then re-dissolved in toluene (5 ml) first and then in hexane (5 ml); the crude was concentrated to give the white carboxylic chloride (0.62 g, 98% yield) as a solid.
5-(5-Bromothiophen-2-yl)-4-cyano-1-(2,4-dichlorophenyl)-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide (5a). A solution in dichloromethane of the carboxylic chloride obtained as described above (2 ml, 0.5 M), was added dropwise to a solution of 1-aminopiperidine (0.13 g, 1.21 mmol) and triethylamine (171 μl, 01.21 mmol) in dichloromethane (2 ml) at 0 °C. The reaction mixture was allowed to reach room temperature and stirred for 16 h. The reaction was quenched with water and the organic phase was extracted with dichloromethane (3 × 3 ml). The combined extracts were washed with brine, dried over anhydrous Na2SO4, filtered, and evaporated under reduced pressure. Flash column chromatography on silica gel (n-hexane/ethyl acetate 5
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) gave carboxamide 5a as a white solid (36% yield). 1H NMR (400 MHz, chloroform-d) δ 7.60 (dd, J = 2.0, 0.5 Hz, 1H), 7.49 (dd, J = 8.5, 2.1 Hz, 2H), 7.45 (d, J = 8.8 Hz, 1H), 7.40 (d, J = 4.0 Hz, 1H), 7.05 (d, J = 4.1 Hz, 1H), 2.88 (t, J = 5.4 Hz, 4H), 1.73 (p, J = 5.7 Hz, 4H), 1.43 (dt, J = 10.3, 5.3 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 156.1, 148.1, 144.7, 138.4, 134.0, 133.9, 131.0, 130.9, 130.8, 128.8, 126.8, 118.1, 112.4, 92.6, 56.8, 25.3, 23.2. ESMS, calculated m/z C20H16BrCl2N5OS 524 [M + H]+, found m/z (relative intensity) 523.9 [M + H]+ (63). HRMS m/z [M + H]+ calcd for C20H17BrCl2N5OS: 523.9714; found: 523.9703.
5) gave carboxamide 5a as a white solid (36% yield). 1H NMR (400 MHz, chloroform-d) δ 7.60 (dd, J = 2.0, 0.5 Hz, 1H), 7.49 (dd, J = 8.5, 2.1 Hz, 2H), 7.45 (d, J = 8.8 Hz, 1H), 7.40 (d, J = 4.0 Hz, 1H), 7.05 (d, J = 4.1 Hz, 1H), 2.88 (t, J = 5.4 Hz, 4H), 1.73 (p, J = 5.7 Hz, 4H), 1.43 (dt, J = 10.3, 5.3 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 156.1, 148.1, 144.7, 138.4, 134.0, 133.9, 131.0, 130.9, 130.8, 128.8, 126.8, 118.1, 112.4, 92.6, 56.8, 25.3, 23.2. ESMS, calculated m/z C20H16BrCl2N5OS 524 [M + H]+, found m/z (relative intensity) 523.9 [M + H]+ (63). HRMS m/z [M + H]+ calcd for C20H17BrCl2N5OS: 523.9714; found: 523.9703.
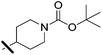
tert-Butyl 4-(5-(5-bromothiophen-2-yl)-4-cyano-1-(2,4-dichlorophenyl)-1H-pyrazole-3-carboxamido)piperidine-1-carboxylate (5b). 5b (92 mg, 51% yield) was obtained as a white solid. 1H NMR (400 MHz, chloroform-d) δ 7.62 (d, J = 2.2 Hz, 1H), 7.49 (dd, J = 8.5, 2.2 Hz, 1H), 7.44 (d, J = 8.5 Hz, 1H), 7.42 (d, J = 4.0 Hz, 1H), 7.06 (d, J = 4.1 Hz, 1H), 6.62 (d, J = 8.0 Hz, 1H), 4.20–4.00 (m, 4H), 2.91 (t, J = 12.8 Hz, 2H), 2.01 (dd, J = 12.9, 4.0 Hz, 2H), 1.45 (s, 9H), 1.39 (dd, J = 11.8, 4.2 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 158.1, 154.8, 148.6, 145.0, 138.6, 134.1, 134.0, 131.1, 130.9, 128.9, 127.0, 118.2, 112.7, 92.3, 79.8, 77.2, 47.1, 42.7, 32.0, 28.5. ESMS, calculated m/z C25H24BrCl2N5O3S 624 [M + H]+, found m/z (relative intensity) 646.0 [M + Na]+ (54). HRMS m/z [M + H]+ calcd for C25H25BrCl2N5O3S: 624.0239; found: 624.0235.
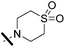
5-(5-Bromothiophen-2-yl)-4-cyano-1-(2,4-dichlorophenyl)-N-(1,1-dioxido-thiomorpholino)-1H-pyrazole-3-carboxamide (5c). 5c (281 mg, 62% yield) was obtained as a white solid. 1H NMR (400 MHz, chloroform-d) δ 7.93 (s, 1H), 7.63 (d, J = 2.2 Hz, 1H), 7.50 (dd, J = 8.5, 2.2 Hz, 1H), 7.43 (d, J = 4.1 Hz, 1H), 7.42 (d, J = 8.4 Hz, 1H), 7.08 (d, J = 4.1 Hz, 1H), 3.58 (t, J = 5.5 Hz, 4H), 3.25 (t, J = 5.3 Hz, 4H); 13C NMR (101 MHz, CDCl3) δ 156.9, 147.0, 145.0, 138.7, 133.9, 133.7, 131.2, 131.0, 130.7, 128.9, 126.5, 118.5, 112.2, 92.4, 52.8, 51.1. ESMS, calculated m/z C19H14BrCl2N5O3S2 574 [M + H]+, found m/z (relative intensity) 573.9 [M + H]+ (57). HRMS m/z M + NH4+ calcd for C19H15BrCl2N5O3S2: 590.9442; found: 590.9434.
5-(5-Bromothiophen-2-yl)-4-cyano-1-(2,4-dichlorophenyl)-N-(fluoromethyl)-1H-pyrazole-3-carboxamide (5d). 5d (119 mg, 32%) was obtained as a white solid. 1H NMR (400 MHz, chloroform-d) δ 7.62 (dd, J = 2.2, 0.4 Hz, 1H), 7.50 (dd, J = 8.5, 2.2 Hz, 1H), 7.44 (d, J = 0.4 Hz, 1H), 7.43 (d, J = 4.0 Hz, 1H), 7.10 (t, J = 6.4 Hz, 1H), 7.07 (d, J = 4.1 Hz, 1H), 4.60 (dt, J = 47.2, 4.8 Hz, 2H), 3.78 (dq, J = 27.9, 5.2 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 158.8, 148.2, 144.9, 138.5, 134.0, 133.9, 131.0, 130.9, 130.7, 128.8, 126.8, 118.2, 112.5, 92.1, 82.43 (d, J = 167.5 Hz), 39.82 (d, J = 19.9 Hz); 19F NMR (376 MHz, chloroform-d) δ −224.10 (tt, J = 47.3, 27.8 Hz). ESMS, calculated m/z C17H10BrCl2FN4OS 487 [M + H]+, found m/z (relative intensity) 486.9 [M + H]+ (56). HRMS m/z [M + H]+ calcd for C17H11BrCl2FN4OS: 486.9198; found: 486.9188.
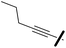
Ethyl 4-cyano-1-(2,4-dichlorophenyl)-5-(5-(pent-1-yn-1-yl)thiophen-2-yl)-1H-pyrazole-3-carboxylate (6a). To a solution of the ester 3 (1.00 g, 1.91 mmol) in diisopropyl amine (8 ml) in a sealed vial, magnetically stirred at 40 °C, bis(triphenylphosphine)-palladium(II)dichloride (0.01 g, 0.01 mmol), triphenyl phosphine (0.01 g, 0.02 mmol) and copper(I) iodide (0.01 g, 0.02 mmol) were added. The reaction mixture was left stirring for 40 min and then the alkyne 1-pentyne (571 μl, 5.73 mmol) was added in one portion. The mixture was warmed up to 80 °C and stirred for 16 h. The reaction was cooled to room temperature, quenched with a solution of HCl 2N and the organic phase was extracted with EtOAc (3 × 7). The combined organic phases were dried over anhydrous Na2SO4, and concentrated under reduced pressure. The black crude was purified by column chromatography on silica gel (Hex/EtOAc 70
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) to afford the alkyne 6 as a pale yellow solid (88%). 1H NMR (400 MHz, chloroform-d) δ 7.58 (dd, J = 1.6, 1.0 Hz, 1H), 7.50 (d, J = 4.0 Hz, 1H), 7.46–7.44 (m, 2H), 7.06 (d, J = 4.0 Hz, 1H), 4.52 (q, J = 7.1 Hz, 2H), 2.39 (t, J = 7.1 Hz, 2H), 1.61 (h, J = 7.3 Hz, 2H), 1.46 (t, J = 7.1 Hz, 3H), 1.01 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 159.4, 145.9, 145.1, 138.2, 134.2, 134.0, 131.5, 130.9, 130.8, 130.4, 129.4, 128.6, 124.6, 112.7, 98.8, 93.2, 72.6, 62.4, 21.8, 21.7, 14.2, 13.6. ESMS, calculated m/z C22H17Cl2N3O2S 458 [M + H]+, found m/z (relative intensity) 458.0 [M + H]+ (100).
30) to afford the alkyne 6 as a pale yellow solid (88%). 1H NMR (400 MHz, chloroform-d) δ 7.58 (dd, J = 1.6, 1.0 Hz, 1H), 7.50 (d, J = 4.0 Hz, 1H), 7.46–7.44 (m, 2H), 7.06 (d, J = 4.0 Hz, 1H), 4.52 (q, J = 7.1 Hz, 2H), 2.39 (t, J = 7.1 Hz, 2H), 1.61 (h, J = 7.3 Hz, 2H), 1.46 (t, J = 7.1 Hz, 3H), 1.01 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 159.4, 145.9, 145.1, 138.2, 134.2, 134.0, 131.5, 130.9, 130.8, 130.4, 129.4, 128.6, 124.6, 112.7, 98.8, 93.2, 72.6, 62.4, 21.8, 21.7, 14.2, 13.6. ESMS, calculated m/z C22H17Cl2N3O2S 458 [M + H]+, found m/z (relative intensity) 458.0 [M + H]+ (100).
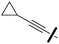
Ethyl 4-cyano-5-(5-(cyclopropylethynyl)thiophen-2-yl)-1-(2,4-dichlorophenyl)-1H-pyrazole-3-carboxylate (6b). 6b (547 mg, 90% yield) was obtained as a white solid. 1H NMR (400 MHz, chloroform-d) δ 7.57 (dd, J = 1.7, 0.8 Hz, 1H), 7.51 (d, J = 4.0 Hz, 1H), 7.46–7.44 (m, 2H), 7.04 (d, J = 4.0 Hz, 1H), 4.51 (q, J = 7.1 Hz, 2H), 1.46 (t, J = 7.1 Hz, 3H), 1.51–1.39 (m, 1H), 0.94–0.86 (m, 2H), 0.86–0.80 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 159.4, 145.9, 145.1, 138.2, 134.2, 134.1, 131.7, 130.9, 130.8, 130.4, 129.4, 128.6, 124.6, 112.7, 101.9, 93.1, 67.7, 62.4, 14.2, 9.0, 0.4. ESMS, calculated m/z C22H15Cl2N3O2S 456 [M + H]+, found m/z (relative intensity) 456.0 [M + H]+ (100).
Ethyl 4-cyano-5-(5-(cyclopentylethynyl)thiophen-2-yl)-1-(2,4-dichlorophenyl)-1H-pyrazole-3-carboxylate (6c). 6c (581 mg, 90% yield) was obtained as a white solid. 1H NMR (400 MHz, chloroform-d) δ 7.58 (dd, J = 1.6, 0.9 Hz, 1H), 7.48 (d, J = 4.0 Hz, 1H), 7.45 (d, J = 1.6 Hz, 2H), 7.04 (d, J = 4.0 Hz, 1H), 4.52 (q, J = 7.1 Hz, 2H), 2.82 (p, J = 7.6 Hz, 1H), 2.03–1.92 (m, 2H), 1.79–1.56 (m, 6H), 1.46 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 159.4, 145.9, 145.1, 138.2, 134.2, 134.0, 131.4, 130.9, 130.7, 130.4, 129.5, 128.6, 124.5, 112.7, 103.0, 93.1, 72.0, 62.4, 33.5, 30.9, 25.1, 14.2. ESMS, calculated m/z C24H19Cl2N3O2S 484 [M + H]+, found m/z (relative intensity) 484.0 [M + H]+ (100).
4-Cyano-1-(2,4-dichlorophenyl)-5-(5-(pent-1-yn-1-yl)thiophen-2-yl)-1H-pyrazole-3-carboxylic acid (7a). To a solution of bromo ester 6a (0.60 g, 1.24 mmol) in methanol (20 ml) was added a solution of potassium hydroxide (0.6 g, 1.24 mmol) in methanol (41 ml) dropwise, at room temperature. The reaction mixture was stirred for 3 h. After the hydrolysis was complete, the reaction mixture was poured into ice-water and acidified with 2N hydrochloric acid. The precipitate was filtered, washed with water, and dried under vacuum to give the acid 7a (84%) as a white solid. 1H NMR (400 MHz, chloroform-d) δ 7.60 (dd, J = 1.7, 0.8 Hz, 1H), 7.53 (d, J = 4.0 Hz, 1H), 7.50–7.44 (m, 2H), 7.07 (d, J = 4.0 Hz, 1H), 2.39 (t, J = 7.1 Hz, 2H), 1.61 (h, J = 7.3 Hz, 2H), 1.01 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 163.3, 145.6, 144.7, 138.4, 134.0, 133.9, 131.6, 130.8, 130.6, 129.7, 128.7, 124.3, 112.3, 99.0, 93.6, 72.6, 53.4, 21.7, 13.6. ESMS, calculated m/z C20H13Cl2N3O2S 430 [M + H]+, found m/z (relative intensity) 468.0 [M + K]+ (100).
4-Cyano-5-(5-(cyclopropylethynyl)thiophen-2-yl)-1-(2,4-dichlorophenyl)-1H-pyrazole-3-carboxylic acid (7b). 7b (380 mg, 90% yield) was obtained as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 14.13 (s, 1H), 8.03 (d, J = 2.2 Hz, 1H), 7.92 (d, J = 8.5 Hz, 1H), 7.76 (dd, J = 8.5, 2.3 Hz, 1H), 7.38 (d, J = 4.0 Hz, 1H), 7.27 (d, J = 4.0 Hz, 1H), 1.59 (tt, J = 8.3, 5.0 Hz, 1H), 0.95–0.88 (m, 2H), 0.80–0.74 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 161.6, 145.1, 144.9, 137.9, 133.6, 133.5, 131.2, 130.4, 130.3, 130.1, 129.0, 128.2, 124.0, 112.1, 101.5, 92.8, 67.2, 50.1, 8.5. ESMS, calculated m/z C20H11Cl2N3O2S 428 [M + H]+, found m/z (relative intensity) 466.0 [M + K]+ (100).
4-Cyano-5-(5-(cyclopentylethynyl)thiophen-2-yl)-1-(2,4-dichlorophenyl)-1H-pyrazole-3-carboxylic acid (7c). 7c (380 mg, 85% yield) was obtained as a white solid. 1H NMR (400 MHz, chloroform-d) δ 7.60 (dd, J = 1.7, 0.8 Hz, 1H), 7.51 (d, J = 4.0 Hz, 1H), 7.50–7.44 (m, 2H), 7.05 (d, J = 4.0 Hz, 1H), 2.82 (p, J = 7.6 Hz, 1H), 2.03–1.93 (m, 2H), 1.79–1.55 (m, 6H); 13C NMR (101 MHz, CDCl3) δ 163.2, 145.6, 144.8, 138.4, 134.0, 133.9, 131.5, 130.8, 130.8, 130.6, 129.8, 128.7, 124.2, 112.3, 103.2, 93.5, 72.0, 33.5, 30.9, 25.1. ESMS, calculated m/z C22H15Cl2N3O2S 456 [M + H]+, found m/z (relative intensity) 494.0 [M + K]+ (100).
4-Cyano-1-(2,4-dichlorophenyl)-5-(5-(pent-1-yn-1-yl)thiophen-2-yl)-1H-pyrazole-3-carbonyl chloride. A solution of the acid 6a (0.15 g, 0.33 mmol) and thionyl chloride (73 μl, 0.99 mmol) in toluene (2.2 ml) was refluxed for 3 h. Solvent was evaporated under reduced pressure and the residue was then re-dissolved in toluene (3 ml) first and then in hexane (3 ml); the crude was concentrated to give the white carboxylic chloride (98% yield) as a solid.
4-Cyano-1-(2,4-dichlorophenyl)-5-(5-(pent-1-yn-1-yl)thiophen-2-yl)-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide (8a). A solution in dichloromethane of the carboxylic chloride obtained as described above (613 μl, 0.7 M), was added dropwise to a solution of 1-aminopiperidine (50 μl, 0.45 mmol) and triethylamine (63 μl, 0.45 mmol) in dichloromethane (0.9 ml) at 0 °C. The reaction mixture was allowed to reach room temperature and stirred for 16 h. The reaction was quenched with water and the organic phase was extracted with dichloromethane (3 × 3 ml). The combined extracts were washed with brine, dried over anhydrous Na2SO4, filtered, and evaporated under reduced pressure. Flash column chromatography on silica gel (n-hexane/ethyl acetate 6
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) gave carboxamide 8a (48% yield). 1H NMR (400 MHz, chloroform-d) δ 7.60 (d, J = 2.1 Hz, 1H), 7.52 (d, J = 4.0 Hz, 1H), 7.48 (dd, J = 8.5, 2.0 Hz, 2H), 7.43 (d, J = 8.4 Hz, 1H), 7.05 (d, J = 4.0 Hz, 1H), 2.89 (t, J = 5.4 Hz, 4H), 2.38 (t, J = 7.1 Hz, 2H), 1.74 (p, J = 5.5 Hz, 4H), 1.60 (h, J = 7.4 Hz, 2H), 1.43 (s, 2H), 1.01 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 156.2, 148.1, 145.2, 138.2, 134.2, 134.0, 131.5, 130.9, 130.8, 130.4, 129.2, 128.7, 124.7, 112.6, 98.7, 92.4, 72.7, 56.8, 25.4, 23.2, 21.8, 21.7, 13.6. ESMS, calculated m/z C25H23Cl2N5OS 475 [M + H]+, found m/z (relative intensity) 475.0 [M + H]+ (100). HRMS m/z [M + H]+ calcd for C25H24Cl2N5OS: 512.1079; found: 512.1066.
4) gave carboxamide 8a (48% yield). 1H NMR (400 MHz, chloroform-d) δ 7.60 (d, J = 2.1 Hz, 1H), 7.52 (d, J = 4.0 Hz, 1H), 7.48 (dd, J = 8.5, 2.0 Hz, 2H), 7.43 (d, J = 8.4 Hz, 1H), 7.05 (d, J = 4.0 Hz, 1H), 2.89 (t, J = 5.4 Hz, 4H), 2.38 (t, J = 7.1 Hz, 2H), 1.74 (p, J = 5.5 Hz, 4H), 1.60 (h, J = 7.4 Hz, 2H), 1.43 (s, 2H), 1.01 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 156.2, 148.1, 145.2, 138.2, 134.2, 134.0, 131.5, 130.9, 130.8, 130.4, 129.2, 128.7, 124.7, 112.6, 98.7, 92.4, 72.7, 56.8, 25.4, 23.2, 21.8, 21.7, 13.6. ESMS, calculated m/z C25H23Cl2N5OS 475 [M + H]+, found m/z (relative intensity) 475.0 [M + H]+ (100). HRMS m/z [M + H]+ calcd for C25H24Cl2N5OS: 512.1079; found: 512.1066.
tert-Butyl 4-(4-cyano-1-(2,4-dichlorophenyl)-5-(5-(pent-1-yn-1-yl)thiophen-2-yl)-1H-pyrazole-3-carboxamido)piperidine-1-carboxylate (8b). 8b (118 mg, 66% yield) was obtained as a white solid. 1H NMR (400 MHz, chloroform-d) δ 7.61 (d, J = 2.2 Hz, 1H), 7.53 (d, J = 3.9 Hz, 1H), 7.48 (dd, J = 8.5, 2.2 Hz, 1H), 7.43 (d, J = 8.5 Hz, 1H), 7.05 (d, J = 4.0 Hz, 1H), 6.62 (d, J = 8.1 Hz, 1H), 4.22–3.96 (m, 4H), 2.91 (t, J = 12.8 Hz, 2H), 2.38 (t, J = 7.1 Hz, 2H), 2.01 (d, J = 12.5 Hz, 2H), 1.60 (h, J = 7.3 Hz, 2H), 1.45 (s, 9H), 1.39 (dd, J = 12.0, 3.8 Hz, 1H), 1.01 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 158.0, 154.7, 148.5, 145.3, 138.3, 134.1, 131.5, 130.9, 130.8, 130.4, 129.2, 128.7, 124.7, 112.8, 98.8, 92.0, 79.7, 72.6, 46.9, 42.7, 31.9, 28.4, 21.8, 21.7, 13.6. ESMS, calculated m/z C30H31Cl2N5O3S 612 [M + H]+, found m/z (relative intensity) 634.1 [M + Na]+ (100). HRMS m/z [M + H]+ calcd for C30H32Cl2N5O3S: 612.1603; found: 612.1607.
4-Cyano-1-(2,4-dichlorophenyl)-N-(1,1-dioxidotetrahydro-2H-thiopyran-4-yl)-5-(5-(pent-1-yn-1-yl)thiophen-2-yl)-1H-pyrazole-3-carboxamide (8c). 8c (98 mg, 46% yield) was obtained as a white solid. 1H NMR (400 MHz, chloroform-d) δ 7.97 (s, 1H), 7.61 (d, J = 2.2 Hz, 1H), 7.52 (d, J = 4.0 Hz, 1H), 7.49 (dd, J = 8.5, 2.2 Hz, 1H), 7.42 (d, J = 8.5 Hz, 1H), 7.05 (d, J = 4.0 Hz, 1H), 3.64–3.52 (m, 4H), 3.24 (t, J = 5.3 Hz, 4H), 2.38 (t, J = 7.1 Hz, 2H), 1.60 (h, J = 7.3 Hz, 2H), 1.00 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 156.9, 147.0, 145.5, 138.5, 134.0, 131.6, 131.0, 130.7, 130.6, 129.6, 128.8, 124.3, 112.4, 99.0, 92.3, 72.6, 52.8, 51.1, 21.8, 21.7, 13.6. ESMS, calculated m/z C24H21Cl2N5O3S2 562 [M + H]+, found m/z (relative intensity) 584.0 [M + Na]+ (100). HRMS m/z [M + H]+ calcd for C24H22Cl2N5O3S2: 562.0541; found: 562.0531.
4-Cyano-1-(2,4-dichlorophenyl)-N-(2-fluoroethyl)-5-(5-(pent-1-yn-1-yl)thiophen-2-yl)-1H-pyrazole-3-carboxamide (8d). 8d (20 mg, 33% yield) was obtained as a white solid. 1H NMR (400 MHz, chloroform-d) δ 7.61 (d, J = 2.2 Hz, 1H), 7.53 (d, J = 4.0 Hz, 1H), 7.48 (dd, J = 8.5, 2.2 Hz, 1H), 7.43 (d, J = 8.5 Hz, 1H), 7.12–7.07 (m, 1H), 7.05 (d, J = 4.0 Hz, 1H), 4.60 (dt, J = 47.3, 4.8 Hz, 2H), 3.78 (dq, J = 28.0, 5.2, 4.8 Hz, 2H), 2.38 (t, J = 7.1 Hz, 2H), 1.61 (h, J = 7.3 Hz, 2H), 1.01 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 158.9, 148.1, 145.3, 138.3, 134.1, 134.0, 131.5, 130.9, 130.8, 130.4, 129.3, 128.7, 124.7, 112.8, 98.8, 92.0, 82.43 (d, J = 167.5 Hz), 72.6, 39.79 (d, J = 20.0 Hz), 21.8, 21.7, 13.6; 19F NMR (376 MHz, chloroform-d) δ −224.06 (tt, J = 47.3, 27.8 Hz). ESMS, calculated m/z C22H17Cl2FN4OS 475 [M + H]+, found m/z (relative intensity) 475.0 [M + H]+ (100). HRMS m/z [M + H]+ calcd for C22H18Cl2FN4OS: 475.0562; found: 475.0551.
4-Cyano-5-(5-(cyclopropylethynyl)thiophen-2-yl)-1-(2,4-dichlorophenyl)-1H-pyrazole-3-carbonyl chloride. A solution of the acid 6b (0.12 g, 0.27 mmol) and thionyl chloride (59 μl, 0.80 mmol) in toluene (1.8 ml) was refluxed for 3 h. Solvent was evaporated under reduced pressure and the residue was then re-dissolved in toluene (3 ml) first and then in hexane (3 ml); the crude was concentrated to give the white carboxylic chloride (97% yield) as a solid.
4-Cyano-5-(5-(cyclopropylethynyl)thiophen-2-yl)-1-(2,4-dichlorophenyl)-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide (8e). A solution in dichloromethane of the carboxylic chloride obtained as described above (515 μl, 0.5 M), was added dropwise to a solution of 1-aminopiperidine (35 μl, 0.31 mmol) and triethylamine (44 μl, 0.31 mmol) in dichloromethane (0.6 ml) at 0 °C. The reaction mixture was allowed to reach room temperature and stirred for 16 h. The reaction was quenched with water and the organic phase was extracted with dichloromethane (3 × 3 ml). The combined extracts were washed with brine, dried over anhydrous Na2SO4, filtered, and evaporated under reduced pressure. Flash column chromatography on silica gel (n-hexane/ethyl acetate 5
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) gave carboxamide 8e as a white solid (50% yield). 1H NMR (400 MHz, chloroform-d) δ 7.60 (d, J = 2.2 Hz, 1H), 7.53 (d, J = 4.0 Hz, 1H), 7.50 (s, 1H), 7.48 (dd, J = 8.5, 2.2 Hz, 1H), 7.42 (d, J = 8.4 Hz, 1H), 7.03 (d, J = 4.0 Hz, 1H), 2.94–2.84 (m, 4H), 1.75 (p, J = 5.6 Hz, 4H), 1.49–1.39 (m, 3H), 0.94–0.77 (m, 4H); 13C NMR (101 MHz, CDCl3) δ 156.2, 148.1, 145.2, 138.2, 134.2, 134.1, 131.7, 130.9, 130.4, 129.2, 128.7, 124.7, 112.6, 101.8, 92.4, 67.7, 56.8, 25.4, 23.2, 9.0, 0.4. ESMS, calculated m/z C25H21Cl2N5OS 510 [M + H]+, found m/z (relative intensity) 510.0 [M + H]+ (100). HRMS m/z [M + H]+ calcd for C25H22Cl2N5OS: 510.0922; found: 510.0906.
5) gave carboxamide 8e as a white solid (50% yield). 1H NMR (400 MHz, chloroform-d) δ 7.60 (d, J = 2.2 Hz, 1H), 7.53 (d, J = 4.0 Hz, 1H), 7.50 (s, 1H), 7.48 (dd, J = 8.5, 2.2 Hz, 1H), 7.42 (d, J = 8.4 Hz, 1H), 7.03 (d, J = 4.0 Hz, 1H), 2.94–2.84 (m, 4H), 1.75 (p, J = 5.6 Hz, 4H), 1.49–1.39 (m, 3H), 0.94–0.77 (m, 4H); 13C NMR (101 MHz, CDCl3) δ 156.2, 148.1, 145.2, 138.2, 134.2, 134.1, 131.7, 130.9, 130.4, 129.2, 128.7, 124.7, 112.6, 101.8, 92.4, 67.7, 56.8, 25.4, 23.2, 9.0, 0.4. ESMS, calculated m/z C25H21Cl2N5OS 510 [M + H]+, found m/z (relative intensity) 510.0 [M + H]+ (100). HRMS m/z [M + H]+ calcd for C25H22Cl2N5OS: 510.0922; found: 510.0906.
tert-Butyl 4-(4-cyano-5-(5-(cyclopropylethynyl)thiophen-2-yl)-1-(2,4-dichlorophenyl)-1H-pyrazole-3-carboxamido)piperidine-1-carboxylate (8f). 8f (95 mg, 54% yield) was obtained as a white solid. 1H NMR (400 MHz, chloroform-d) δ 7.61 (d, J = 2.2 Hz, 1H), 7.53 (d, J = 4.0 Hz, 1H), 7.48 (dd, J = 8.5, 2.2 Hz, 1H), 7.43 (d, J = 8.5 Hz, 1H), 7.03 (d, J = 4.0 Hz, 1H), 6.61 (d, J = 8.1 Hz, 1H), 4.20–3.98 (m, 4H), 2.91 (t, J = 12.8 Hz, 2H), 2.01 (d, J = 14.4 Hz, 2H), 1.45 (s, 9H), 1.42 (dd, J = 8.7, 3.5 Hz, 2H), 0.93–0.78 (m, 4H); 13C NMR (101 MHz, CDCl3) δ 158.2, 154.8, 148.6, 145.4, 138.4, 134.3, 134.2, 131.8, 131.0, 130.9, 130.5, 129.3, 128.8, 124.8, 112.9, 102.0, 92.1, 79.8, 67.8, 47.1, 42.8, 32.0, 28.5, 9.1, 0.6. ESMS, calculated m/z C30H29Cl2N5O3S 610 [M + H]+, found m/z (relative intensity) 632.1 [M + Na]+ (100). HRMS m/z [M + H]+ calcd for C30H30Cl2N5O3S: 610.1446; found: 610.1444.
4-Cyano-5-(5-(cyclopropylethynyl)thiophen-2-yl)-1-(2,4-dichlorophenyl)-N-(1,1-dioxidotetrahydro-2H-thiopyran-4-yl)-1H-pyrazole-3-carboxamide (8g). 8g (71 mg, 40% yield) was obtained as a white solid. 1H NMR (400 MHz, chloroform-d) δ 7.92 (s, 1H), 7.62 (d, J = 2.2 Hz, 1H), 7.54 (d, J = 4.0 Hz, 1H), 7.49 (dd, J = 8.5, 2.2 Hz, 1H), 7.41 (d, J = 8.5 Hz, 1H), 7.04 (d, J = 4.0 Hz, 1H), 3.64–3.53 (m, 4H), 3.29–3.20 (m, 4H), 1.45 (tt, J = 8.1, 5.0 Hz, 1H), 0.95–0.78 (m, 4H); 13C NMR (101 MHz, CDCl3) δ 156.9, 147.0, 145.5, 138.5, 134.0, 133.9, 131.8, 131.0, 130.7, 130.6, 129.5, 128.8, 124.3, 112.4, 102.1, 92.2, 67.6, 52.9, 51.1, 9.0, 0.5. ESMS, calculated m/z C24H19Cl2N5O3S2 560 [M + H]+, found m/z (relative intensity) 582.1 [M + Na]+ (100). HRMS m/z [M + H]+ calcd for C24H20Cl2N5O3S2: 560.0385; found: 560.0370.
4-Cyano-5-(5-(cyclopropylethynyl)thiophen-2-yl)-1-(2,4-dichlorophenyl)-N-(2-fluoroethyl)-1H-pyrazole-3-carboxamide (8h). 8h (58 mg, 28% yield) was obtained as a white solid. 1H NMR (400 MHz, chloroform-d) δ 7.61 (d, J = 2.1 Hz, 1H), 7.54 (d, J = 4.0 Hz, 1H), 7.48 (dd, J = 8.5, 2.2 Hz, 1H), 7.43 (d, J = 8.8 Hz, 1H), 7.09 (t, J = 6.1 Hz, 1H), 7.03 (dd, J = 4.0, 0.4 Hz, 1H), 4.60 (dt, J = 47.3, 4.7 Hz, 2H), 3.77 (dq, J = 27.7, 4.9 Hz, 2H), 1.45 (tt, J = 8.2, 5.1 Hz, 1H), 0.94–0.79 (m, 4H); 13C NMR (101 MHz, CDCl3) δ 158.9, 148.1, 145.3, 138.3, 134.1, 134.0, 131.7, 130.9, 130.8, 130.4, 129.3, 128.7, 124.7, 112.8, 101.8, 92.0, 82.44 (d, J = 167.5 Hz), 67.7, 39.79 (d, J = 19.9 Hz), 9.0, 0.44; 19F NMR (376 MHz, chloroform-d) δ −224.06 (tt, J = 47.2, 27.8 Hz). ESMS, calculated m/z C22H15Cl2FN4OS 472.0 [M + H]+, found m/z (relative intensity) 474 [M + H]+ (100). HRMS m/z [M + H]+ calcd for C22H16Cl2FN4OS: 473.0406; found: 473.0394.
4-Cyano-5-(5-(cyclopentylethynyl)thiophen-2-yl)-1-(2,4-dichlorophenyl)-1H-pyrazole-3-carbonyl chloride. A solution of the acid 6c (0.10 g, 0.21 mmol) and thionyl chloride (46 μl, 0.62 mmol) in toluene (2.2 ml) was refluxed for 3 h. Solvent was evaporated under reduced pressure and the residue was then re-dissolved in toluene (3 ml) first and then in hexane (3 ml); the crude was concentrated to give the white carboxylic chloride (96% yield) as a solid.
4-Cyano-5-(5-(cyclopentylethynyl)thiophen-2-yl)-1-(2,4-dichlorophenyl)-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide (8i). A solution in dichloromethane of the carboxylic chloride obtained as described above (578 μl, 0.4 M), was added dropwise to a solution of 1-aminopiperidine (30 μl, 0.27 mmol) and triethylamine (38 μl, 0.27 mmol) in dichloromethane (0.5 ml) at 0 °C. The reaction mixture was allowed to reach room temperature and stirred for 16 h. The reaction was quenched with water and the organic phase was extracted with dichloromethane (3 × 3 ml). The combined extracts were washed with brine, dried over anhydrous Na2SO4, filtered, and evaporated under reduced pressure. Flash column chromatography on silica gel (n-hexane/ethyl acetate 6
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) gave carboxamide 8i as a white solid (43% yield). 1H NMR (400 MHz, chloroform-d) δ 7.60 (d, J = 2.2 Hz, 1H), 7.50 (d, J = 4.0 Hz, 2H), 7.48 (dd, J = 8.4, 2.2 Hz, 1H), 7.43 (d, J = 8.5 Hz, 1H), 7.03 (d, J = 4.0 Hz, 1H), 2.94–2.86 (m, 4H), 2.81 (p, J = 7.6 Hz, 1H), 2.04–1.90 (m, 2H), 1.81–1.54 (m, 10H), 1.49–1.24 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 156.2, 148.1, 145.2, 138.2, 134.2, 134.0, 131.5, 130.9, 130.4, 129.3, 128.7, 124.6, 112.6, 102.9, 92.4, 72.1, 56.8, 33.6, 31.0, 25.4, 25.1, 23.2. ESMS, calculated m/z C27H25Cl2N5OS 538 [M + H]+, found m/z (relative intensity) 538.1 [M + H]+ (17). HRMS m/z [M + H]+ calcd for C27H26Cl2N5OS: 538.1235; found: 538.1220.
4) gave carboxamide 8i as a white solid (43% yield). 1H NMR (400 MHz, chloroform-d) δ 7.60 (d, J = 2.2 Hz, 1H), 7.50 (d, J = 4.0 Hz, 2H), 7.48 (dd, J = 8.4, 2.2 Hz, 1H), 7.43 (d, J = 8.5 Hz, 1H), 7.03 (d, J = 4.0 Hz, 1H), 2.94–2.86 (m, 4H), 2.81 (p, J = 7.6 Hz, 1H), 2.04–1.90 (m, 2H), 1.81–1.54 (m, 10H), 1.49–1.24 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 156.2, 148.1, 145.2, 138.2, 134.2, 134.0, 131.5, 130.9, 130.4, 129.3, 128.7, 124.6, 112.6, 102.9, 92.4, 72.1, 56.8, 33.6, 31.0, 25.4, 25.1, 23.2. ESMS, calculated m/z C27H25Cl2N5OS 538 [M + H]+, found m/z (relative intensity) 538.1 [M + H]+ (17). HRMS m/z [M + H]+ calcd for C27H26Cl2N5OS: 538.1235; found: 538.1220.
tert-Butyl 4-(4-cyano-5-(5-(cyclopentylethynyl)thiophen-2-yl)-1-(2,4-dichlorophenyl)-1H-pyrazole-3-carboxamido)piperidine-1-carboxylate (8j). 8j (75 mg, 48% yield) was obtained as a white solid. 1H NMR (400 MHz, chloroform-d) δ 7.61 (d, J = 2.1 Hz, 1H), 7.51 (d, J = 4.0 Hz, 1H), 7.48 (dd, J = 8.5, 2.2 Hz, 1H), 7.43 (d, J = 8.4 Hz, 1H), 7.04 (d, J = 4.0 Hz, 1H), 6.62 (d, J = 8.1 Hz, 1H), 4.21–3.99 (m, 4H), 2.91 (t, J = 12.7 Hz, 2H), 2.81 (p, J = 7.6 Hz, 1H), 2.05–1.93 (m, 4H), 1.81–1.56 (m, 6H), 1.45 (s, 9H), 1.39 (dd, J = 12.6, 3.5 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 158.0, 154.7, 148.5, 145.3, 138.2, 134.2, 134.0, 131.5, 130.9, 130.8, 130.4, 129.4, 128.7, 124.6, 112.9, 102.9, 92.0, 79.7, 72.0, 46.9, 42.8, 33.6, 31.9, 31.0, 28.4, 25.1. ESMS, calculated m/z C32H33Cl2N5O3S 638 [M + H]+, found m/z (relative intensity) 660.1 [M + Na]+ (70). HRMS m/z [M + H]+ calcd for C32H34Cl2N5O3S: 638.1759; found: 638.1750.
4-Cyano-5-(5-(cyclopentylethynyl)thiophen-2-yl)-1-(2,4-dichlorophenyl)-N-(1,1-dioxidotetrahydro-2H-thiopyran-4-yl)-1H-pyrazole-3-carboxamide (8k). 8k (50 mg, 39% yield) was obtained as a white solid. 1H NMR (400 MHz, chloroform-d) δ 7.99 (s, 1H), 7.61 (d, J = 4.0 Hz, 1H), 7.53–7.40 (m, 3H), 7.03 (d, J = 4.1 Hz, 1H), 3.59 (d, J = 10.6 Hz, 4H), 3.24 (s, 4H), 2.81 (p, J = 7.4 Hz, 1H), 1.97 (s, 2H), 1.81–1.50 (m, 6H); 13C NMR (101 MHz, CDCl3) δ 157.0, 147.0, 145.5, 138.4, 134.0, 133.9, 131.5, 131.0, 130.7, 130.6, 129.7, 128.8, 124.3, 112.4, 103.2, 92.3, 72.0, 52.8, 51.1, 33.6, 31.0, 25.1. ESMS, calculated m/z C26H23Cl2N5O3S2 588 [M + H]+, found m/z (relative intensity) 610 [M + Na]+ (40). HRMS m/z [M + H]+ calcd for C26H24Cl2N5O3S2: 588.0698; found: 588.0685.
4-Cyano-5-(5-(cyclopentylethynyl)thiophen-2-yl)-1-(2,4-dichlorophenyl)-N-(2-fluoroethyl)-1H-pyrazole-3-carboxamide (8l). 8l (15 mg, 31% yield) was obtained as a white solid. 1H NMR (400 MHz, chloroform-d) δ 7.61 (d, J = 2.2 Hz, 1H), 7.51 (d, J = 4.0 Hz, 1H), 7.49 (dd, J = 8.5, 2.2 Hz, 1H), 7.43 (d, J = 8.4 Hz, 1H), 7.09 (t, J = 6.1 Hz, 1H), 7.04 (d, J = 4.0 Hz, 1H), 4.60 (dt, J = 47.2, 4.7 Hz, 2H), 3.78 (dq, J = 27.5, 5.1, 4.6 Hz, 2H), 2.81 (p, J = 7.5 Hz, 1H), 2.03–1.89 (m, 2H), 1.81–1.56 (m, 6H); 13C NMR (101 MHz, CDCl3) δ 158.9, 148.1, 145.4, 138.2, 134.2, 134.0, 131.4, 130.9, 130.8, 130.4, 129.4, 128.7, 124.6, 112.8, 102.9, 92.0, 82.44 (d, J = 167.5 Hz), 72.0, 39.79 (d, J = 19.9 Hz), 33.6, 31.0, 25.1; 19F NMR (376 MHz, chloroform-d) δ −224.06 (tt, J = 47.2, 27.8 Hz). ESMS, calculated m/z C24H19Cl2FN4OS 501 [M + H]+, found m/z (relative intensity) 501.0 [M + H]+ (100). HRMS m/z [M + H]+ calcd for C24H20Cl2FN4OS: 501.0719; found: 501.0706.
Determination of the partition coefficients
Measurement of chromatographic capacity factor (k′) by C-18 HPLC method was performed using the equation k′ = (tr − t0)/t0,17,18 where t0 is the retention time of unretained substance and tr is the compound's retention time. The mobile phase was prepared mixing methanol with water in proportions of 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 and the flow rate was 1 ml min−1. A solution of urea in a methanol–water solvent (85
15 and the flow rate was 1 ml min−1. A solution of urea in a methanol–water solvent (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) was used for measurement of the column dead time (t0 = 2582 ± 0.005, n = 3). Seven compounds having known log
15) was used for measurement of the column dead time (t0 = 2582 ± 0.005, n = 3). Seven compounds having known log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values, tabulated and in the range from 1.10 to 5.70 (benzyl alcohol, log
P values, tabulated and in the range from 1.10 to 5.70 (benzyl alcohol, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 1.10; benzene, log
P 1.10; benzene, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 2.10; toluene, log
P 2.10; toluene, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 2.70; naphthalene, log
P 2.70; naphthalene, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 3.60; biphenyl, log
P 3.60; biphenyl, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 4; phenanthrene, log
P 4; phenanthrene, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 4.50; triphenylamine, log
P 4.50; triphenylamine, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 5.70) were chosen as a “standard” calibration mixture for the determination of retention times (tr). Every measure was obtained in triplicate and at controlled temperature of 25 °C. Capacity factors (k′) were calculated. The log
P 5.70) were chosen as a “standard” calibration mixture for the determination of retention times (tr). Every measure was obtained in triplicate and at controlled temperature of 25 °C. Capacity factors (k′) were calculated. The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k′ value for each of seven compounds was plotted against its relative lipophilicity value reported in literature, based on the established linear relationship (log
k′ value for each of seven compounds was plotted against its relative lipophilicity value reported in literature, based on the established linear relationship (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 2.60 log
P = 2.60 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k′ + 3.58, correlation coefficient = 0.92). The capacity factor of each compound was determined (the value was obtained on average of three experiments) and the relative log
k′ + 3.58, correlation coefficient = 0.92). The capacity factor of each compound was determined (the value was obtained on average of three experiments) and the relative log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P value was obtained by extrapolation.
P value was obtained by extrapolation.
Equilibrium binding assays
Binding assays were performed with the CB1 receptor agonist, [3H]CP55940 (0.7 nM), 1 mg ml−1 bovine serum albumin (BSA) and 50 mM Tris buffer containing 0.1 mM EDTA and 0.5 mM MgCl2 (pH 7.4), total assay volume 500 μl. Binding was initiated by the addition of mouse brain membranes (30 μg) or CB2 transfected CHO cells (5 μg). Assays were carried out at 37 °C for 60 minutes before termination by addition of ice-cold wash buffer (50 mM Tris buffer, 1 mg ml−1 BSA) and vacuum filtration using a 24-well sampling manifold (Brandel Cell Harvester) and Whatman GF/B glass-fibre filters that had been soaked in wash buffer at 4 °C for 24 h. Each reaction tube was washed five times with a 4 ml aliquot of buffer. The filters were oven-dried for 60 min and then placed in 5 ml of scintillation fluid (Ultima Gold XR, Packard), and radioactivity quantitated by liquid scintillation spectrometry. Specific binding was defined as the difference between the binding that occurred in the presence and absence of 1 μM of the corresponding unlabelled ligand and was 70–80% of the total binding.[35S]GTPγS binding assay
Mouse brain membranes (5 μg protein) were preincubated for 30 minutes at 30 °C with adenosine deaminase (0.5 U ml−1). The membranes were then incubated with the agonist with vehicle or modulator for 60 minutes at 30 °C in assay buffer (50 mM Tris–HCl; 50 mM Tris-base; 5 mM MgCl2; 1 mM EDTA; 100 mM NaCl; 1 mM DTT; 0.1% BSA) in the presence of 0.1 nM [35S]GTPγS and 30 μM GDP, in a final volume of 500 μl. Binding was initiated by the addition of [35S]GTPγS. Nonspecific binding was measured in the presence of 30 μM GTPγS. The reaction was terminated by rapid vacuum filtration (50 mM Tris–HCl; 50 mM Tris-base; 0.1% BSA) using a 24-well sampling manifold (cell harvester; Brandel, Gaitherburg, MD) and GF/B filters (Whatman, Maidstone, UK) that had been soaked in buffer (50 mM Tris–HCl; 50 mM Tris-Base; 0.1% BSA) for at least 24 hours. Each reaction tube was washed six times with a 1.2 ml aliquot of ice-cold wash buffer. The filters were oven-dried for at least 60 minutes and then placed in 5 ml of scintillation fluid (Ultima Gold XR, Packard). Radioactivity was quantified by liquid scintillation spectrometry.Acknowledgements
We thank the European Commission for financial support (Industry Academia Partnerships and Pathways project “PET BRAIN”, Contract no 251482). The EPSRC National Mass Spectrometry Service Centre (Swansea, UK) is gratefully acknowledged for performing HRMS analyses. S.A. thanks the Northern Research Partnership (http://www.northscotland-research.ac.uk/) and Pharmaness (http://www.pharmaness.it) for co-funding a PhD studentship.Notes and references
- R. J. Chorvat, Bioorg. Med. Chem. Lett., 2013, 23, 4751–4760 CrossRef CAS PubMed
.
- G. Kunos, D. Osei-Hyiaman, S. Bátkai, K. A. Sharkey and A. Makriyannis, Trends Pharmacol. Sci., 2009, 30, 1–7 CrossRef CAS PubMed
.
- A. Serrano, F. J. Pavon, J. Suarez, M. Romero-Cuevas, E. Baixeras, P. Goya and F. R. de Fonseca, Current Obesity Reports, 2012, vol. 1, pp. 216–228 Search PubMed
.
- S.-L. Tseng, M.-S. Hung, C.-P. Chang, J.-S. Song, C.-L. Tai, H.-H. Chiu, W.-P. Hsieh, Y. Lin, W.-L. Chung, C.-W. Kuo, C.-H. Wu, C.-M. Chu, Y.-S. Tung, Y.-S. Chao and K.-S. Shia, J. Med. Chem., 2008, 51, 5397–5412 CrossRef CAS PubMed
.
- M.-S. Hung, C.-P. Chang, T.-C. Li, T.-K. Yeh, J.-S. Song, Y. Lin, C.-H. Wu, P.-C. Kuo, P. K. Amancha, Y.-C. Wong, W.-C. Hsiao, Y.-S. Chao and K.-S. Shia, ChemMedChem, 2010, 5, 1439–1443 CrossRef CAS PubMed
.
- H. Fan, H. T. Ravert, D. P. Holt, R. F. Dannals and A. G. Horti, J. Labelled Compd. Radiopharm., 2006, 49, 1021–1036 CrossRef CAS
.
- M. Gao, M. Wang and Q.-H. Zheng, Bioorg. Med. Chem. Lett., 2012, 22, 3704–3709 CrossRef CAS PubMed
.
- R. A. Ross, H. C. Brockie, L. A. Stevenson, V. L. Murphy, F. Templeton, A. Makriyannis and R. G. Pertwee, Br. J. Pharmacol., 1999, 126, 665–672 CrossRef CAS PubMed
.
- J. L. Wiley, R. G. Jefferson, M. C. Grier, A. Mahadevan, R. K. Razdan and B. R. Martin, J. Pharmacol. Exp. Ther., 2001, 296, 1013–1022 CAS
.
- Z. Li, A. Gifford, Q. Liu, R. Thotapally, Y.-S. Ding, A. Makriyannis and S. J. Gatley, Nucl. Med. Biol., 2005, 32, 361–366 CrossRef CAS PubMed
.
- S. A. Hitchcock and L. D. Pennington, J. Med. Chem., 2006, 49, 7559–7583 CrossRef CAS PubMed
.
- R. Distinto, MSc, Università degli studi di Cagliari, 2009
.
- Online Calculator Plugins were used for structure property prediction and calculation. Marvin version 6.0.5, 2013, http://www.chemaxon.com/marvin/sketch/index.php, ChemAxon (http://www.chemaxon.com).
- A. G. Horti, H. Fan, H. Kuwabara, J. Hilton, H. T. Ravert, D. P. Holt, M. Alexander, A. Kumar, A. Rahmim, U. Scheffel, D. F. Wong and R. F. Dannals, J. Nucl. Med., 2006, 47, 1689–1696 CAS
.
- N. Cluny, V. Vemuri, A. Chambers, C. Limebeer, H. Bedard, J. Wood, B. Lutz, A. Zimmer, L. Parker, A. Makriyannis and K. Sharkey, Br. J. Pharmacol., 2010, 161, 629–642 CrossRef CAS PubMed
.
- W. C. Still, M. Kahn and A. Mitra, J. Org. Chem., 1978, 43, 2923–2925 CrossRef CAS
.
- Organisation for Economic Co-operation and Development, Test No. 117: Partition Coefficient (n-octanol/water), HPLC Method, OECD Publishing, Paris, 2004 Search PubMed
.
- X. Fei and Q. Zheng, J. Liq. Chromatogr. Relat. Technol., 2005, 28, 939–945 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H, 13C, 19F NMR spectra of all the new compounds, ligand displacement and functional assays curves. See DOI: 10.1039/c4ra17274d |
| This journal is © The Royal Society of Chemistry 2015 |