A novel approach to size separation of gold nanoparticles by capillary electrophoresis–evaporative light scattering detection
Abstract
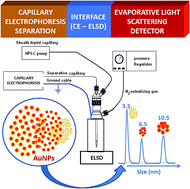
A simple and rapid methodology to separate and characterize gold nanoparticles (AuNPs) in aqueous medium by capillary electrophoresis–evaporative light scattering detection (CE–ELSD) is presented. First, a controlled synthesis procedure to obtain water-soluble AuNPs, by varying the trisodium citrate concentration was described. These free AuNPs were separated by capillary zone electrophoresis (CZE) based on the differences in the charge-to-mass ratio of the AuNPs–citrate in a mixed buffer of ammonium acetate (20 mM), containing tris(hydroxymethyl)-aminomethane (Tris, 20 mM) and 3-(cyclohexylamino)-1-propanesulfonic acid (CAPS; 10 mM) at pH 8.5. Under optimal working conditions, three small different-sized AuNPs were successfully separated whose average sizes were 3.5, 6.5 and 10.5 nm. The average diameter was lower than 1.2 nm for all of them (calculated by high-resolution transmission electron microscopy, TEM). Thus, this CE-based method was able to separate AuNPs that differ by only 3 nm in diameter. It can be a valuable methodology for the rapid and cost-effective characterization of other nanomaterials in the future in aqueous solutions.

 Please wait while we load your content...
Please wait while we load your content...