Preparation of imidazolium-type ionic liquids containing silsesquioxane frameworks and their thermal and ion-conductive properties
Abstract
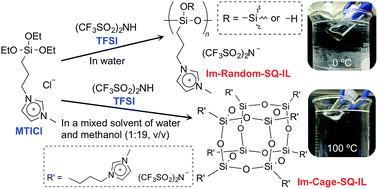
An ionic liquid containing a random-structured oligosilsesquioxane (Im-Random-SQ-IL) was successfully prepared by the hydrolytic condensation of 1-methyl-3-[3-(triethoxysilyl)propyl]imidazolium chloride (MTICl) in aqueous bis(trifluoromethanesulfonyl)imide (TFSI). Im-Random-SQ-IL exhibited a glass transition temperature (Tg) at −25 °C as indicated by an endothermic peak in the differential scanning calorimetry (DSC) curve. In addition, fluidity was visually observed at ca. 0 °C, i.e. Im-Random-SQ-IL is a room temperature ionic liquid. Conversely, when the hydrolytic condensation of MTICl was performed using a water/methanol (1 : 19 v/v) solution of TFSI, an ionic liquid containing a cage-like oligosilsesquioxane (Im-Cage-SQ-IL) was obtained. The Tg of Im-Cage-SQ-IL was −22 °C, and its melting temperature (Tm) was 105 °C according to the DSC analysis. In addition, fluidity was observed for this ionic liquid at ca. 100 °C. These results suggest that both the amorphous structure of Im-Random-SQ-IL and the type of substituent groups in the silsesquioxane contributed to the ionic liquid behaviour below room temperature. In addition, these ionic liquids exhibited high thermal stabilities (Im-Random-SQ-IL: Td3 = 429 °C, Td5 = 437 °C and Td10 = 447 °C, Im-Cage-SQ-IL: Td3 = 427 °C, Td5 = 436 °C and Td10 = 446 °C) and relatively high ion conductivities (10−4–10−3 S cm−1, at ∼100 °C).

 Please wait while we load your content...
Please wait while we load your content...