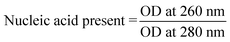N-doped carbon nanosheets with antibacterial activity: mechanistic insight†
Amlan Chakraborty‡
a,
Pranav Patni‡a,
Deepa Suhag‡a,
Gajender Sainib,
Anirudha Singhc,
Sandip Chakrabarti*d and
Monalisa Mukherjee*a
aBiomimetic and Nanostructured Materials Research Laboratory, Amity Institute of Biotechnology, Amity University Uttar Pradesh, Sector-125, Noida, India. E-mail: mmukherjee@amity.edu
bAdvance Instrumentation Research Facility, Jawaharlal Nehru University, New Delhi, India
cFaculty of Translational Tissue Engineering Center & Dept. of Urology, Johns Hopkins School of Medicine, Baltimore, Maryland Area, USA
dAmity Institute of Nanotechnology, Amity University Uttar Pradesh, Sector-125, Noida, India. E-mail: schakrabarti@amity.edu
First published on 24th February 2015
Abstract
The rising incidence of drug resistant diseases has led to an increasing need for developing novel and efficient antimicrobial products that can counter these infections. We report for the first time, the exceptional antibacterial activity of N-doped carbon nanosheets (CNS). The antibacterial activity and mechanism of action of CNS was examined for gram negative E. coli. Based on the cell viability tests, nucleic acid quantitation, time and concentration dependent antibacterial activity tests and SEM and TEM micrographs, performed under similar concentration and incubation conditions, the CNS dispersion shows the highest antibacterial activity, sequentially followed by GO, rGO and CCM, with a loss of cell viability by 92.1 ± 1.7%. We envision that the physical stress and piercing action caused by sharp “knife-edges” as well as the presence of heteroatoms in CNS result in the rupturing of the bacterial cell wall, eventually causing cell death. The high ID/IG ratio (0.99) of CNS is closely related to the formation of structural and edge plane defects, especially in the case of N-doped carbonaceous materials, which is one of the key factors in enhancing the antibacterial activity of the material.
Introduction
In recent years, the rising number of drug resistant microorganisms has led to an increasing need to develop effective antimicrobial products that can combat these strains. Several carbon materials, such as carbon nanotubes (CNT),1–10 carbon nanofibers (CNF),11,12 carbon nanoparticles (CNP),13–17 graphene oxide (GO),18,19 and reduced graphene oxide (rGO),20,21 have found their use in water treatment,22 microfluidics,23 chemical and biological sensors,24,25 separation membranes,26 energy storage,27 improved accessibility of reactants to the active sites,28 and hydrogel29 along with various other applications. Moreover, carbon materials have also been extensively employed for antibacterial applications. Among them, GO and rGO have been established to have notable antibacterial activity.30The antibacterial activity of GO and rGO is attributed to the sharp edge planes present in GO sheets which create membrane stress. This leads to physical damage of the bacterial cell membrane resulting in the loss of membrane integrity and RNA leakage.31,32 However, according to some groups, cells trapped within the sheets maintain their structural integrity but are unable to proliferate in the media, thereby inhibiting cellular growth.33 Furthermore, another mechanism by which GO acts on the bacteria and kills them is by inducing oxidative stress which occurs due to the release of reactive oxygen species.30
Dispersed graphene based materials are known to display strong antibacterial activity.30 Several other factors influence the antibacterial activity of carbon materials, which includes the interaction between carbon materials and bacterial cells, such as incubation time,34 concentration,35 medium,36 and light sources.37
Carbon nanosheets (CNS), which comprise multi-layered graphene films are a new class of carbon material and possess high surface to volume ratio, sharp edge plane defects and lightness with higher efficacy compared to graphene.38–40 This is because CNS is made up of fewer graphite layers.41,42
Conventional antibiotics function by inhibiting the formation of cell wall, nucleic acid synthesis and proteins.43–45 However, to the best of our knowledge, the antibacterial activity of CNS has not yet been explored. N-doping could also enhance the biocompatibility of carbon nanomaterials. The presence of amino groups may be the reason for better biocompatibility of the N-doped carbon materials when compared to undoped carbon materials.46 Further, applications of CNS as an antibacterial material and other practical applications warrant the understanding of the interaction mechanism.
Herein, we report for the first time the antibacterial activity and the mechanism of CNS towards gram negative E. coli. In this study we do the comparative analysis of antibacterial activity of CNS along with graphene based materials (GO and rGO) and the undoped carbonized carbon material (CCM) towards a gram negative bacterial model, E. coli. The time and concentration dependent antibacterial activities were examined. Scanning electron microscopy and transmission electron microscopy were applied to show the behaviour of CNS towards the bacterial cells. The coating of CNS over flexible substrates like cellulose acetate paper for antibacterial and water purification purpose has potential applications in hospitals and health care.
Experimental
Materials
Glycerol and melamine were purchased from M/S Sigma-Aldrich. Concentrated sulphuric acid (98%) was purchased from M/S Merck, Germany. Luria Bertani (LB) media was purchased from M/S Merck, Germany. E. coli K-12 was procured from Medox, Bangalore, India. All chemicals except melamine were used as received without further purifications.Characterization
Synthesis
CNS was synthesized by improved literature procedure. Melamine was purified prior to use for the synthesis of CNS. Melamine was dissolved in aqueous solution of caustic soda at 130 °C. The resultant hot caustic liquor was then clarified by decantation or filtration. The solution was further cooled to room temperature, giving rise to crystallized, substantially pure melamine product. This purified melamine was employed for CNS synthesis. Solvothermal technique was adapted for synthesis of CNS material following the reported synthetic procedure.47 Nitrogen was incorporated in CNS using melamine as one of the starting materials. In a typical synthetic procedure, 0.5 g of melamine and 10 ml of glycerol were mixed together and stirred until melamine was totally dissolved into glycerol, followed by addition of 10 ml of 98% sulphuric acid under vigorous stirring. The mixture was then transferred into a 50 ml Teflon-lined autoclave and heated at 180 °C for 4 h under autogeneous pressure. The obtained black solid product was then washed with Milli-Q water and ethanol three times to remove the impurities. It was further dried at 50 °C in a hot air oven for an hour. The FESEM images revealed that the product was devoid of any carbon spheres and comprised of only thin sheets, unlike the product obtained from previously reported procedure.Nitrogen free coke-like carbonaceous material (CCM) was synthesized using glycerol and sulphuric acid. In a typical procedure, 10 ml of 98% sulphuric acid was added to 10 ml of glycerol and stirred vigorously. The mixture was then transferred into a 50 ml Teflon-lined autoclave and heated at 180 °C for 4 h under autogeneous pressure. The resulting black colored powder was then washed with ethanol followed by deionized (DI) water. The synthesized CCM was dried for 1 h at 50 °C in a hot air oven.
GO was synthesized using a modified version of Hummer's method.48 Graphite powder (1 g) was added to 25 ml of concentrated H2SO4 and the mixture was cooled to 0 °C (on ice). Then, 3 g of KMnO4 was gradually added (temperature was maintained at around <10 °C throughout the reaction) and was stirred continuously. The mixture was then allowed to cool at room temperature. To this reaction mixture, 50 ml of DI H2O was added and the temperature was seen to rise up to 55 °C. Then, 150 ml of DI H2O was added again and was stirred continuously for 30 minutes. Post stirring, 15 ml of H2O2 was added to the mixture and stirred for another 30 minutes. Black colored saturated slurry was seen to be formed. After saturation, the slurry was vacuum filtered and was washed with 5% HCl until the supernatant turned colorless. The resulting solid material obtained was left to dry in ambient conditions.
rGO was synthesized using reported procedures.48 In a typical procedure, 20 mg of GO was dispersed in 10 ml of DI H2O. This dispersion was sonicated for 1 h using a Telesonic ultrasonic bath cleanser. The sonicated mixture was then taken in a PTFE lined stainless steel autoclave and heated at 120 °C for 4 h under autogeneous pressure. The mixture was then washed with acetone and water. The resulting solid was allowed to dry overnight in ambient conditions.
GO and rGO were characterized as per reported methods (data not shown).48,49
Cell preparation
50 μl of E. coli K-12 was spread and grown in LB agar plates at 37 °C. A single colony was hence inoculated in LB medium at 37 °C to obtain the culture. By checking the optical density at 600 nm the mid log growth phase was determined. The culture was then centrifuged at 6000 rpm for 10 minutes to pellet down the cells and washed thrice in 0.9% (w/v) saline solution. The pellets were re-suspended in fresh LB medium. Bacterial cell suspensions were diluted to obtain cell samples containing 106 to 107 CFU ml−1.Cell viability test
Concentration dependent and time dependent antibacterial activity of CNS along with graphene based materials GO and rGO were studied towards E. coli cells. For this purpose, E. coli cells were incubated with freshly synthesized CNS, CCM, GO and rGO suspensions (separately) in LB medium at 37 °C under 180 rpm shaking speed for 3 h. The concentration of CNS, CCM, GO and rGO was taken as 5, 10, 20, 50, and 100 μg ml−1 respectively. The viability of E. coli cells was evaluated by the colony counting method. A series of 7 fold cell dilutions (100 μl each) were spread on to LB agar plates and left to grow overnight (20 h) at 37 °C. A control plate was also made with 100 μl cell suspension devoid of CNS or any graphene based materials. Colonies were counted and compared to those on control plate and hence calculating the change in the cell growth inhibition.Loss of viability was calculated using the following formula:
In a similar manner, a time dependent study was also conducted, taking 20 μg ml−1 as the concentration for CNS/CCM/GO/rGO.
E. coli cells were incubated with freshly synthesized CNS, CCM, GO and rGO suspensions (separately) in LB medium at 37 °C under 180 rpm shaking speed for 3 h at a concentration of 20 μg ml−1. At every half an hour interval, cells with a series of 7 fold cell dilutions (100 μl each) were spread on to LB agar plates and left to grow overnight (20 h) at 37 °C. The viability of E. coli cells was evaluated by the colony counting method. A control plate was also made with 100 μl cell suspension devoid of CNS or any graphene based materials. Colonies were counted and compared to those on control plate and hence calculating the change in the cell growth inhibition. The optical density of the samples after incubation with suspension was taken at half hour intervals, taking LB media as reference. Loss of viability was calculated by using the formula given above.
Nucleic acid quantitation
E. coli cells were incubated with freshly synthesized CNS suspension in LB medium at 37 °C under 180 rpm shaking speed for 3 h at a concentration of 20 μg ml−1. The sample was centrifuged at 5000 rpm for 10 minutes and the supernatant was decanted in a fresh tube. A dilution of 10× (Dilution Factor 10) was done for the supernatant collected. Absorbance at 260 nm (OD260) and absorbance at 280 nm (OD280) was measured for the diluted sample in a UV spectrophotometer. The amount of nucleic acid present was calculated using the following formula:Scanning electron micrograph of gram negative E. coli and E. coli incubated with CNS
Gram negative E. coli (control) and E. coli exposed to CNS (20 μg ml−1) were fixed with 2.5% glutaraldehyde in PBS buffer (pH 7.5), stained with 1% osmium tetraoxide, dehydrated with graded ethanol series (20, 40, 60, 80, 95 and 100% ethanol), embedded on a copper grid, and subsequently sputter coating of gold was carried out on the samples.Transmission electron micrograph of gram negative E. coli and E. coli incubated with CNS
Gram negative E. coli (control) and E. coli exposed to CNS(20 μg ml−1) were fixed with 2.5% glutaraldehyde in PBS buffer (pH 7.5). Cells were washed repeatedly with 1× PBS and were incubated with 1% OsO4 for 1 h. The cells were then dehydrated with graded ethanol series (70% for 15 minutes twice, 90% for 15 minutes twice and finally with 100% ethanol for 15 minutes twice). The cells were then embedded in epon/araldite resin for polymerization at 65 °C for 15 h. After polymerization, the resin was cut to thin sections of 90 nm and was placed on the grids. Staining for 1 minute with 4% Uranyl acetate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1: acetone
1: acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water) and 0.2% Reynolds lead citrate (water) was done. This was followed by air drying the sample and was observed under Transmission Electron microscope (JEOL 2100F) at an acceleration voltage of 120 kV.
water) and 0.2% Reynolds lead citrate (water) was done. This was followed by air drying the sample and was observed under Transmission Electron microscope (JEOL 2100F) at an acceleration voltage of 120 kV.
Results and discussion
Chemical composition
Raman spectra of the synthesized CNS and CCM exhibited G-bands at 1562 and 1574 cm−1 respectively, for CNS and CCM. The presence of D-bands in both the materials suggest the presence of sp2 lattice structure. The D-bands 1346 and 1355 cm−1 respectively, for CNS and CCM indicate presence of disordered structures in both the materials. Significant increase in the ID/IG ratio from 0.86 (CCM) to 0.99 for CNS was attributed to the presence of more disorder and boundaries in CNS compared to the CCM (Fig. 1a). The increased degree of disorderliness in the N-doped CNS is due to the presence of the heteroatom in its carbon structure.50 | ||
| Fig. 1 (a) Raman spectra (at 633 nm laser excitation) of CNS & CCM, and (b) survey XPS spectra of CNS & CCM. | ||
XPS measurements were carried out to reveal more details on the chemical bonding states of both CNS and CCM. The XPS survey spectra over a wide range of binding energies (0–1200 eV) for both CNS and CCM show a predominant narrow C 1s peak at 284.5 eV, O 1s peak at 531.5 eV. N 1s peak at 400.5 eV, specifically in case of CNS, indicating the incorporation of nitrogen in the structure. S 2p peaks were also observed at 167.5 eV. The XPS data were further supported by the elemental analysis of carbon (C), hydrogen (H), nitrogen (N) and oxygen (O) atoms. CNS contains nitrogen whereas, no nitrogen was found in CCM (Fig. 1b). C, H, N and O atom percentages for CNS (N% in CNS is 1.75%) and CCM are provided in Table 1 (please see ESI 1†).
Dispersion analysis
The appearance of all four carbon materials (Fig. 3) vary from each other and this difference in appearance after dispersion may be associated with their respective structural and physicochemical properties. All the four samples (300 μg ml−1) were sonicated in an ultrasonicator for 2 h and allowed to stand for 1 week. It was observed that CCM and rGO particles precipitated after the CCM and rGO suspensions were left to stand still for 6 h. In case of GO, the particles started to precipitate out after 2 days and the suspension appeared yellow-brown in colour. However, the CNS dispersion remained stable even after 1 week, resulting in an almost homogeneously grey-black dispersion. The better dispersion of CNS over GO could be attributed to the presence of amino groups (from melamine) in between the layers of CNS in addition to the other hydrophilic functional groups, like hydroxyl and carboxyl groups being present in its carbon framework.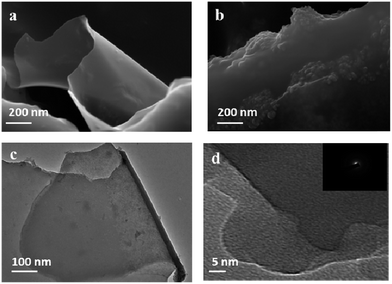 | ||
| Fig. 2 (a) FESEM of CNS, (b) FESEM of CCM, (c) TEM & (d) HRTEM of CNS (SAED pattern of CNS as inset of Fig. 2d). | ||
Morphology and structure of CNS and CCM
The morphology of the as-synthesized CNS and CCM was examined by FESEM, TEM and HRTEM. The FESEM image of CNS revealed thin flower petal like structure (Fig. 2b). The TEM (Fig. 2d) micrograph displayed sheet like structure with folded areas and sharp edges confirming a 2D CNS material. The absence of a noticeable lattice structure on the enlarged HRTEM (Fig. 2c) revealed that the CNS material is disordered or amorphous. The diffused ring patterns obtained from the Selected Area Electron Diffraction (SAED; inset Fig. 2d) of CNS indicated that the CNS material was amorphous, which was further confirmed by the broad diffraction peaks observed in XRD pattern of CNS powder (ESI 2†). The FESEM image of CCM (Fig. 2b) revealed the presence of coke-like carbonaceous blocks devoid of thin sheets.Antibacterial activity
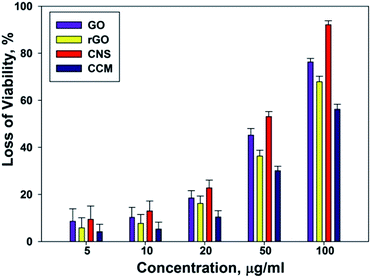
E. coli was used as a model bacterium to evaluate the antibacterial activity of CNS, CCM, GO, rGO. The viability of bacterial cells was estimated by colony counting method as described in materials and methods section. In the concentration dependent study, a range of concentration; 5, 10, 20, 50, and 100 μg ml−1 for CNS, CCM, GO and rGO had been taken. As shown in Fig. 4, CNS displayed stronger antibacterial activity compared to GO and rGO and an increased antibacterial activity in comparison to CCM. However, CNS has proved to be a better inhibitor of bacterial growth at a concentration of 20 μg ml−1. The loss of E. coli viability leaps from 8.6 ± 5.3% at the GO concentration of 5 μg ml−1 to 76.3 ± 1.5% at 100 μg ml−1. In a similar manner, rGO at the concentration of 5 μg ml−1 kills only 5.8 ± 4.3% of E. coli, while 100 μg ml−1 rGO kills 67.9 ± 2.3% of E. coli. In case of CNS, the loss of E. coli viability leaps from 9.4 ± 5.7% at the CNS concentration of 5 μg ml−1 to 92.1 ± 1.7% at 100 μg ml−1. In comparison to CNS, CCM shows a poor antibacterial activity where the loss of E. coli viability jumps from 4.2 ± 3.1% at the CCM concentration of 5 μg ml−1 to 56.2 ± 2.1% at 100 μg ml−1. The majority of E. coli was killed after incubation with CNS at the concentration of 100 μg ml−1 as we infer from Fig. 4.We further examined the time dependent antibacterial activity of the four carbon materials (CNS, CCM, GO and rGO). All the four materials at a concentration of 20 μg ml−1 were incubated separately with E. coli for 3 h. The loss of E. coli viability was counted at half hour intervals. Fig. 5 indicates the loss of E. coli viability, which is significant in the period of 1 h to 2 h of incubation. A rapid increase in the loss of viability was observed after 2 h of incubation. The inhibition observed after 3 h by the three materials over a period of time showed maximum inhibition by CNS, followed by GO then rGO and least by CCM which is 64.9 ± 1.3%, 56.7 ± 1.1%, 43.1 ± 1.8% and 39.6 ± 1.5% respectively.
The findings of the time dependent study were validated by taking absorbance at 600 nm in half hour intervals, with LB medium as reference, since absorbance is proportional to the number of cells. Fig. 6a indicates the effect of GO on bacterial growth where we note that even after 1 h of incubation there is no significant growth observed. This shows the presence of an extended lag phase however short (as compared to CNS Fig. 6d). The inhibition is however limited and there is growth of bacteria with similar growth pattern as that of the control. In case of rGO, Fig. 6b, there is no presence of any extension in the lag phase, however; there is significant growth, although less, as compared to that of control. In case of CCM, Fig. 6c, it was observed that there is poor/negligible inhibition as exhibited by the increasing absorbance due to bacterial growth similar to the control sample which clearly tells that CCM does not interact with the bacteria. Hence, CNS has proved its enhanced antibacterial effect as can be observed from Fig. 6d where we find a prolonged lag phase and there is negligible bacterial growth which lasts for 1.5 h. Subsequently, it was found that the exponential growth after 2 h of incubation was comparatively less as that of the control.
Proposed antibacterial mechanism of CNS on gram negative E. coli
The antibacterial activity results reveal that CNS was able to effectively kill gram negative E. coli with loss of cell viability by 92.1 ± 1.7%. E. coli has a cytoplasmic membrane (∼7 nm), a peptidoglycan layer (∼7–8 nm) and an outer membrane (∼10–15 nm). This inner peptidoglycan along with the outer membrane provides resistance to antibiotics. In our study, we propose the mechanism of action of CNS as an antibacterial agent on gram negative bacteria, E. coli. Possibly, CNS acts as a “knife-edge” and physically punctures the membrane by direct contact, as has been observed from the coloured SEM micrographs (Fig. 7a–d).When a cell is ruptured, its cytoplasmic constituents come out of the cell. This cytoplasmic constituent has been monitored by nucleic acid quantitation where a ratio has been calculated of absorbance at 260 nm to absorbance at 280 nm. The ratio obtained was 1.9, which suggests both the presence of RNA and also DNA in lower amounts. The coloured SEM micrograph (Fig. 7a) shows gram negative E. coli without CNS (taken as control). Fig. 7a inset displays a smooth E. coli membrane surface. SEM morphological observations taken after 1 h of incubation with 20 μg ml−1 CNS (Fig. 7b) clearly indicated (by arrows) the punctured cells, where in some cases, only the outer cell membrane was intact (inset of Fig. 7b). The grey scale surface in the background displays CNS. Upon further incubation up to 2 h, an increased loss of membrane integrity and flaccidity (Fig. 7c) along with membrane distortion was observed. After 3 h of incubation (Fig. 7d) it was seen that almost all the cells have lost their rod-shaped structural integrity. Similar physical piercing and distortions were seen in case of fullerene nanoparticles towards gram positive bacteria.51
These rough knife edges of CNS are also responsible for causing interactions with the exposed peptidoglycan resulting in the loss of integrity between the N-acetyl muramine (NAM) and N-acetyl glucosamine (NAG). This piercing and puncturing action of CNS leads to oozing out of the cytoplasmic constituents (Fig. 9), resulting in cell death.
The TEM micrograph in Fig. 8 further depicts Gram negative E. coli with an outer most lipid membrane, a peptidoglycan layer and innermost cytoplasmic membrane. It was observed that CNS comes in contact with the outer lipid membrane and results in penetrating the cell membrane. TEM images (Fig. 8b and c), suggest that certain portions of the CNS are visible inside the cell membrane and this may be due to the contact-mediated penetrating action of CNS. The outer lipid membrane which is present only in gram negative bacterium is first ruptured followed by interaction and dissociation of the peptidoglycan layer and finally the cell membrane. This leads to oozing out of the cytoplasmic contents (Fig. 8d), and the cells lose its ability to withstand the turgor stress. Leakage of the cellular contents has been confirmed by nucleic acid quantitation. The cell eventually attains a flaccid structure showing all its cytoplasmic contents leaked out (Fig. 8d). The TEM micrograph therefore clearly indicates the possible contact-mediated mechanism of “penetration” followed by “rupturing” of the cell membrane by direct contact.
Conclusions
The present study assessed the antibacterial activity of N-doped CNS for gram negative bacteria. The as-synthesized N-doped CNS displayed extraordinary antibacterial activity towards the gram negative bacteria, E. coli, compared to other carbon materials such as GO, rGO and CCM. The high performance antibacterial activity of CNS could be due to the sharp “knife-edges” present in abundance in CNS which when come in contact with the bacteria result in physical damage of the cell wall by causing membrane stress. It was also observed that the undoped carbon material (CCM) demonstrated very poor antibacterial activity in comparison with the N-doped CNS, confirming the role of nitrogen doping in the enhanced antibacterial activity of CNS. The detailed mechanism by which nitrogen enhances the antibacterial activity is under study. Given the superior antibacterial activity of CNS over other popular carbon based materials, we expect that this new carbon nanomaterial could offer promising potentials in advancement of antibacterial materials.Acknowledgements
This work was supported by Department of Science and Technology (DST), Government of India (SR/FT/CS-006/2010, SERB).References
- H. Chen, B. Wang, D. Gao, M. Guan, L. Zheng, H. Ouyang, Z. Chai, Y. Zhao and W. Feng, Small, 2013, 9, 2735 CrossRef CAS PubMed.
- C. Wu, Polym. Int., 2011, 60, 807 CrossRef CAS.
- R. Mohan, A. M. Shanmugharaj and R. S. Hun, J. Biomed. Mater. Res., Part B, 2011, 96, 119 CrossRef PubMed.
- Y. T. Joo, K. H. Jung, M. J. Kim and Y. Kim, J. Appl. Polym. Sci., 2013, 127, 1508 CrossRef CAS.
- J. Wang, Z. Dong, J. Huang, J. Li, K. Liu, J. Jin and J. Ma, RSC Adv., 2013, 3, 918 RSC.
- I. Kim, T. An, W. Choi, C. S. Kim, H. J. Chac and G. Lim, RSC Adv., 2014, 4, 1347 RSC.
- A. R. Deokar, L. Lin, C. Changcd and Y. Ling, J. Mater. Chem. B, 2013, 1, 2639 RSC.
- B. Das, P. Chattopadhyay, A. Upadhyay, K. Gupta, M. Mandal and N. Karak, New J. Chem., 2014, 38, 4300 RSC.
- S. Liu, L. Wei, L. Hao, N. Fang, M. W. Chang, R. Xu, Y. Yang and Y. Chen, ACS Nano, 2009, 3, 3891 CrossRef CAS PubMed.
- S. Kang, M. Herzberg, D. F. Rodrigues and M. Elimelech, Langmuir, 2008, 24, 6409 CrossRef CAS PubMed.
- A. Oya and S. Yoshida, Carbon, 1996, 34, 53 CrossRef CAS.
- S. J. Park and Y. S. Jang, J. Colloid Interface Sci., 2003, 261, 238 CrossRef CAS.
- X. Cai, M. Lin, S. Tan, W. Mai, Y. Zhang, Z. Liang, Z. Lin and X. Zhang, Carbon, 2012, 50, 3407 CrossRef CAS PubMed.
- T. Kavitha, A. I. Gopalan, K. P. Lee and S. Y. Park, Carbon, 2012, 50, 2994 CrossRef CAS PubMed.
- X. Wang, X. Liu, J. Chen, H. Han and Z. Yuan, Carbon, 2014, 68, 798 CrossRef CAS PubMed.
- X. Liu, Y. Luo, T. Wu and J. Huang, New J. Chem., 2012, 36, 2568 RSC.
- A. Niu, Y. Han, J. Wu, N. Yu and Q. Xu, J. Phys. Chem. C, 2010, 114, 12728 CAS.
- J. Zhao, B. Deng, M. Lv, J. Li, Y. Zhang, H. Jiang, C. Peng, J. Li, J. Shi, Q. Huang and C. Fan, Adv. Healthcare Mater., 2013, 2, 1259 CrossRef CAS PubMed.
- S. Liu, M. Hu, T. H. Zeng, R. Wu, R. Jiang, J. Wei, L. Wang, J. Kong and Y. Chen, Langmuir, 2012, 28, 12364 CrossRef CAS PubMed.
- T. S. Sreeprasad, M. S. Maliyekkal, K. Deepti, K. Chaudhari, P. L. Xavier and T. Pradeep, ACS Appl. Mater. Interfaces, 2011, 3, 2643 CAS.
- S. Kellici, J. Acord, J. Ball, H. S. Reehal, D. Morganc and B. Saha, RSC Adv., 2014, 4, 14858 RSC.
- Y. L. F. Musico, C. M. Santos, M. L. P. Dalida and D. F. Rodrigues, ACS Sustainable Chem. Eng., 2014, 2, 1559 CrossRef CAS.
- I. Kim, T. An, W. Choi, C. S. Kim, H. J. Chac and G. Lim, RSC Adv., 2014, 4, 1347 RSC.
- Y. Zhang, J. Li, Y. Shen, M. Wang and J. Li, J. Phys. Chem. B, 2004, 108, 15343 CrossRef CAS.
- W. C. Poh, K. P. Loh, W. D. Zhang, S. Triparthy, J. S. Ye and F. S. Sheu, Langmuir, 2004, 20, 5484 CrossRef CAS.
- L. Sun and R. M. Crooks, J. Am. Chem. Soc., 2000, 122, 12340 CrossRef CAS.
- C. Park, P. E. Anderson, A. Chambers, C. D. Tan, R. Hidalgo and N. M. Rodriguez, J. Phys. Chem. B, 1999, 103, 10572 CrossRef CAS.
- J. Prabhuram, T. S. Zhao, Z. K. Tang, R. Chen and Z. X. Liang, J. Phys. Chem. B, 2006, 110, 5245 CrossRef CAS PubMed.
- J. Venkatesan, R. Jayakumar, A. Mohandas, I. Bhatnagar and S. K. Kim, Materials, 2014, 7, 3946 CrossRef CAS PubMed.
- S. Liu, T. H. Zeng, M. Hofmann, E. Burcombe, J. Wei, R. Jiang, J. Kong and Y. Chen, ACS Nano, 2011, 5, 6971 CrossRef CAS PubMed.
- O. Akhavan and E. Ghaderi, ACS Nano, 2010, 4, 5731 CrossRef CAS PubMed.
- W. Hu, C. Peng, W. Luo, M. Lv, X. Li, D. Li, Q. Huang and C. Fan, ACS Nano, 2010, 7, 4317 CrossRef PubMed.
- I. E. M. Carpio, J. D. Mangadlao, H. N. Nguyen, R. C. Advincula and D. F. Rodrigues, Carbon, 2014, 77, 289 CrossRef PubMed.
- S. Liu, A. K. Ng, R. Xu, J. Wei, C. M. Tan, Y. Yang and Y. Chen, Nanoscale, 2010, 2, 2744 RSC.
- S. B. Liu, L. Wei, L. Hao, N. Fang, M. W. Chang, R. Xu, Y. H. Yang and Y. Chen, ACS Nano, 2009, 3, 3891 CrossRef CAS PubMed.
- L. R. Arias and L. J. Yang, Langmuir, 2009, 25, 3003 CrossRef CAS PubMed.
- D. Y. Lyon, D. A. Brown and P. J. J. Alvarez, Water Sci. Technol., 2008, 57, 1533 CrossRef CAS PubMed.
- N. G. Shang, P. Papakonstantinou, M. McMullan, M. Chu, A. Stamboulis, A. Potenza, S. S. Dhesi and H. Marchetto, Adv. Funct. Mater., 2008, 18, 3506 CrossRef CAS.
- M. Liu, Y. Yan, L. Zhang, X. Wang and C. Wang, J. Mater. Chem., 2012, 22, 11458 RSC.
- Q. Kuang, S. Y. Xie, Z. Y. Jiang, X. H. Zhang, Z. X. Xie, R. B. Huang and L. S. Zheng, Carbon, 2004, 42, 1737 CrossRef CAS PubMed.
- Y. Wu, B. Yang, B. Zong, H. Sun, Z. Shen and Y. Feng, J. Mater. Chem., 2004, 14, 469 RSC.
- J. M. Shen and Y. T. Feng, J. Phys. Chem. C, 2008, 112, 13114 CAS.
- T. Mathur, V. Kalia, T. K. Barman, S. Singhal, S. Khan, D. J. Upadhyay, A. Rattan and V. S. Raj, Int. J. Antimicrob. Agents, 2013, 41, 36 CrossRef CAS PubMed.
- J. Nakamura, H. Yamashiro, S. Hayashi, M. Yamamoto, K. Miura, S. Xu, T. Doi, H. Maki, O. Yoshida and H. Arimoto, Chem.–Eur. J., 2012, 18, 12681 CrossRef CAS PubMed.
- J. Hoque, P. Akkapeddi, V. Yarlagadda, D. S. S. M. Uppu, P. Kumar and J. Haldar, Langmuir, 2012, 28, 12225 CrossRef CAS PubMed.
- J. C. Carrero-Sanchez, A. L. Elias, R. Mancilla, G. Arrellin, H. Terrones, J. P. Laclette and M. Terrones, Nano Lett., 2006, 6, 1609 CrossRef CAS PubMed.
- W. Wang, S. Chakrabarti, Z. Chen, Z. Yan, M. O. Tade, J. Zou and Q. Li, J. Mater. Chem., 2014, 2, 2390 RSC.
- W. S. O. Hummers and E. Richard, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- C. Nethravathi and M. Rajamathi, Carbon, 2008, 46, 1994 CrossRef CAS PubMed.
- W. S. Tseng, C. Y. Tseng and C. T. Kuo, Nanoscale Res. Lett., 2009, 4, 234 CrossRef CAS PubMed.
- N. Tsao, T. Y. Luh, C. K. Chou, T. Y. Chang, J. J. Wu, C. C. Liu and H. Y. Lei, J. Antimicrob. Chemother., 2002, 49, 641 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: ESI 1: Carbon, hydrogen, nitrogen and oxygen percentages from CNS and CCM. ESI 2: XRD pattern of CNS and CCM. See DOI: 10.1039/c4ra17049k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |