Applications of dynamic electrochemical impedance spectroscopy (DEIS) to evaluate protective coatings formed on AZ31 magnesium alloy
Abstract
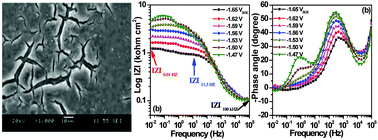
The present investigation explores the possibility of using dynamic electrochemical impedance spectroscopy (DEIS) to evaluate the corrosion protection of conversion coating produced on an AZ31 alloy in simulated body fluid (SBF). The protection extended by the conversion coating was clearly noticed from an increase in DEIS-impedance and phase angle maximum values.

 Please wait while we load your content...
Please wait while we load your content...