Optimisation of the process variables in production of activated carbon by microwave heating
Anirban Kundua,
Bhaskar Sen Guptab,
M. A. Hashimc,
J. N. Sahud,
Mubarak Mujawarce and
Ghufran Redzwan*a
aInstitute of Biological Sciences, University of Malaya, 50603, Kuala Lumpur, Malaysia. E-mail: ghufran@um.edu.my; Fax: +6 0379674178; Tel: +6 0379676797
bWater Academy, School of the Built Environment, Heriot-Watt University, Edinburgh Campus, EH14 4AS, Scotland
cDepartment of Chemical Engineering, University of Malaya, 50603, Kula Lumpur, Malaysia
dDepartment of Petroleum and Chemical Engineering, Faculty of Engineering, Institut Teknologi Brunei, P.O. Box 2909, Tungku Gadong, Brunei Darussalam
eDepartment of Chemical and Petroleum Engineering, Faculty of Engineering, UCSI University, Kuala Lumpur 56000, Malaysia
First published on 10th April 2015
Abstract
This study aims to investigate the optimal operating conditions in order to obtain cost effective production of activated carbon (AC) from palm kernel shell (PKS) by microwave heating. Interactions among the independent variables, namely irradiation time (T), microwave power (W), impregnation ratio between impregnating substances and PKS, and the concentration of impregnating substance (sulphuric acid) were considered for optimising the process parameters during the production of AC, aided by Central Composite Design. The optimum conditions for the independent process variables were 11.02 minutes of irradiation time, microwave power of 676 W and impregnation ratio of 0.68. The AC produced in this work had a surface area of 1011 m2 g−1 with high porosity as shown by scanning electron microscopy (SEM). Zinc was used to verify the potential of AC as an adsorbent. Zinc removal at the optimum conditions was found to be 13.72 mg g−1. Such a Zn removal value is comparable with the earlier work of other researchers who used a conventional way of producing the AC. It is believed that microwave technology can be used for the production of AC in a short time with high energy efficiency, e.g., 11 minutes against 2–5 hours of reactivation for conventional methods.
1. Introduction
Activated carbon (AC) is a unique and versatile adsorbent that has been used since the ancient times by Egyptians and Indians for removal of unwanted odour, taste, dyes, heavy metals and organic substances. In modern times, it has been widely used by the chemical industry for removal, separation and pre-concentration of both metallic and organic species from water and waste water.1–3 There have been persistent efforts to prepare AC from low cost materials4 to reduce the production cost. However, the cost of energy for the production of AC remains a concern. A typical production process of the AC requires heating the carbonaceous materials for one to seven hours to convert them into porous AC.5–9 The requirement for this long heating time may be due to the fact that, heating occurs through convection, conduction, and radiation of thermal energy from the surface of the material towards the core. This creates a bottleneck in the manufacturing process.Thus, the objective of this study was to investigate the optimal operating conditions for cost effective production of AC. The important factors that contribute to production cost are heating time as well energy and chemical use. The use of microwave technology could facilitate cost effective production,10,11 as in the case of microwave heating, the thermal energy is produced due to molecular interaction with the microwave energy. The microwave penetrates the material and the microwave energy is converted to thermal energy. Thus, heat is generated throughout the bulk of the material and distribute more efficiently within the material. This can reduce the processing time and improve the overall product quality.12
Response surface methodology (RSM) is a collection of mathematical and statistical techniques which aid the analysis of experimental data and the optimisation of the result within a given range of operating parameters.13 A Box-Wilson Central Composite Design, more familiar by the name of central composite design (CCD), is one of the most popular response surface methodology (RSM) designs due to the advantages that, CCDs are very efficient in providing important information on effects of the experimental variables and experimental error in a minimum number of experimental runs. The flexibility of CCDs allows studying different experimental regions of interest and operability conditions with the help of several varieties of CCD.14–16
In the conventional one-factor-at a time experiments, number of experiments are very large as well as, no interaction effects are studied. However, in RSM, reaction parameters can be varied simultaneously as required to generate data and that data can be used to propose an empirical model. This makes the RSM a better economical and quick analytical approach towards optimisation than the conventional approach.17 Hence, CCD and RSM methods were used in this study to determine the optimum operating conditions. Palm kernel shells (PKS) were used in this study as the raw material as this material is widely available as agricultural waste in Malaysia. This raw material was impregnated with sulphuric acid. Zinc was chosen as model adsorbate as a response to find out the effectiveness of the AC. Zinc is a carcinogenic heavy metal, and it causes internal organ damage and death to human being if taken in excessive dose. It is also harmful to the aquatic ecosystems.18–20 To understand the physical character of the produced AC at the optimum conditions, scanning electron micrograph (SEM) imaging, Fourier transform infrared spectroscopy (FTIR) and BET surface area were used in this study.
2. Methodology
2.1. Materials used in the preparation of AC
Palm kernel shells (PKS) obtained from a local palm oil mill were washed to remove dust and dried in an oven at 105 °C until its mass became constant. Sulphuric acid and zinc nitrate used in this study were of analytical grade and obtained from R&M chemicals, Malaysia and Fluka Chemicals, UK. All solutions were prepared in distilled water. A stock solution of Zn(II) of concentration 1000 mg L−1 was prepared with zinc nitrate. The desired concentrations were prepared by dilution method from the stock solution with distilled water.2.2. Design of experiment
A CCD method was chosen so that a quadratic surface could be fitted to the response with minimum number of experiments. It was believed that the interaction between the effective process parameters could be identified and finally, the combination of operating parameters would be identified to optimise the response. The most effective parameters namely, irradiation time (T) coded as Factor A, microwave power (P) coded as Factor B, impregnation ratio (IR) coded as Factor C and sulphuric acid concentration coded as Factor D, responsible for the quality of the AC were taken at various levels as marked in Table 1. It shows the ranges and the levels of independent variables in this study. Here the low actual values of the factors were coded as −1 and the high actual values were coded as +1.| Factor | Name | Units | Type | Low actual | High actual | Low coded | High coded | Mean |
|---|---|---|---|---|---|---|---|---|
| A | Time | Min | Numeric | 5 | 20 | −1 | 1 | 12.5 |
| B | Power | W | Numeric | 600 | 1000 | −1 | 1 | 800 |
| C | IR | Numeric | 0.5 | 2 | −1 | 1 | 1.25 | |
| D | Conc. of acid | % | Numeric | 20 | 100 | −1 | 1 | 60 |
The total number of runs in CCD consisted of factorial runs, axial runs and centre runs. Eqn (1) gives the number of total runs required to fit the quadratic surface where n is number of independent variables.
| N = 2n + 2n + nc | (1) |
In this study, a face-centred CCD for four variables, consisting of 16 factorial points, eight axial points and four replicates at the centre points with a total number of 28 experiments was applied to find the optimum condition. The four replicate experiments at centre point were used for estimating the experimental error and the duplicability of the data. Table 2 shows the different combinations of the independent variables suggested by the Design Expert software (version 7.1.6, Stat-Ease, Inc.)21,22 as well as the response for each run.
| Std | Run | Type | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Adsorption (mg g−1) | Yield (%) |
|---|---|---|---|---|---|---|---|---|
| A: time min | B: power W | C: IR | D: conc. of acid% | |||||
| 4 | 1 | Fact | 20 | 1000 | 0.5 | 20 | 1.305 | 90.17 |
| 6 | 2 | Fact | 20 | 600 | 2 | 20 | 4.895 | 58.65 |
| 28 | 3 | Center | 12.5 | 800 | 1.25 | 60 | 12.599 | 62.25 |
| 21 | 4 | Axial | 12.5 | 800 | 0.5 | 60 | 11.568 | 77.61 |
| 22 | 5 | Axial | 12.5 | 800 | 2 | 60 | 14.6955 | 49.7 |
| 1 | 6 | Fact | 5 | 600 | 0.5 | 20 | 3.445 | 93.64 |
| 9 | 7 | Fact | 5 | 600 | 0.5 | 100 | 6.9585 | 97.3 |
| 10 | 8 | Fact | 20 | 600 | 0.5 | 100 | 9.9105 | 68.62 |
| 16 | 9 | Fact | 20 | 1000 | 2 | 100 | 5.852 | 31.26 |
| 24 | 10 | Axial | 12.5 | 800 | 1.25 | 100 | 14.807 | 69.99 |
| 17 | 11 | Axial | 5 | 800 | 1.25 | 60 | 7.7605 | 78.59 |
| 8 | 12 | Fact | 20 | 1000 | 2 | 20 | 5.265 | 53.19 |
| 20 | 13 | Axial | 12.5 | 1000 | 1.25 | 60 | 11.8895 | 44.91 |
| 7 | 14 | Fact | 5 | 1000 | 2 | 20 | 6.905 | 85.03 |
| 11 | 15 | Fact | 5 | 1000 | 0.5 | 100 | 3.5015 | 66.06 |
| 26 | 16 | Center | 12.5 | 800 | 1.25 | 60 | 14.112 | 61.99 |
| 5 | 17 | Fact | 5 | 600 | 2 | 20 | 6.21 | 82.85 |
| 27 | 18 | Center | 12.5 | 800 | 1.25 | 60 | 14.787 | 48.95 |
| 19 | 19 | Axial | 12.5 | 600 | 1.25 | 60 | 14.6085 | 59.06 |
| 18 | 20 | Axial | 20 | 800 | 1.25 | 60 | 11.9525 | 51.15 |
| 23 | 21 | Axial | 12.5 | 800 | 1.25 | 20 | 12.285 | 74.73 |
| 25 | 22 | Center | 12.5 | 800 | 1.25 | 60 | 14.666 | 48.86 |
| 14 | 23 | Fact | 20 | 600 | 2 | 100 | 12.9625 | 39.45 |
| 2 | 24 | Fact | 20 | 600 | 0.5 | 20 | 5.14 | 82.94 |
| 3 | 25 | Fact | 5 | 1000 | 0.5 | 20 | 3.115 | 94.74 |
| 15 | 26 | Fact | 5 | 1000 | 2 | 100 | 8.958 | 70.66 |
| 13 | 27 | Fact | 5 | 600 | 2 | 100 | 4.926 | 91.28 |
| 12 | 28 | Fact | 20 | 1000 | 0.5 | 100 | 5.443 | 53.76 |
Yield of AC (y1) and the adsorption of zinc (y2) were taken as the response of the processes. ANOVA, regression analysis and the interaction among the parameters were also studied. A second order polynomial eqn (2) was used to model the relation between the process variables and the response.23,24
 | (2) |
2.3. Preparation of the adsorbent
The raw material was grinded to 1000–2000 μm size and washed thoroughly with distilled water. The grinded PKSs were impregnated with different concentration of sulphuric acid at different impregnation ratios. Undiluted sulphuric acid was considered as 100% that was subsequently diluted as per requirement. Twenty grams of the precursor was mixed with the required amount of acid for four hours with constant stirring at 120 rpm at room temperature. The slurry was then dried in a vacuum oven at 100 °C for 24 h. A microwave furnace with frequency 2450 MHz (SYNOTHERM corporation, model: HAMiLab-C) was used for heating purpose. The impregnated and dried samples were placed in a specially designed quartz tube, which was placed vertically in the microwave oven for the required length of time and at the required microwave power, based on the experimental design. The quartz tube was purged with nitrogen gas at a flow of 0.4 L m−1 for 5 minutes before microwave treatment to outgas air. This flow rate was maintained during the activation and cooling stages. The product obtained was then washed thoroughly with distilled water until the pH of the washing solution reached a value between 5 and 6.2.4. Experimental work
Batch adsorption experiments were conducted to eliminate volume correction. Experiments were performed in 250 mL Erlenmeyer flasks containing 100 mL of the solution and constantly agitated at 120 rpm until equilibrium was reached. All adsorption experiments were performed at pH 5 to eliminate the chance of precipitation. Hydrochloric acid was used to maintain the pH of the solution.After the reaction, all samples were filtered with ‘Filtres Fioroni 601’ filter paper and the final concentration of zinc was measured with ICP-OES (Perkin-Elmer 7000DV). The following equation was applied to calculate the final concentration of zinc,
 | (3) |
2.5. Characterisation of the surface
The pore structures of the prepared AC were analysed using N2 adsorption and scanning electron microscopy (SEM). Nitrogen adsorption/desorption isotherms were used to measure the BET surface area, total pore volume and pore size distribution at 77 K by a Quantachrome Autosorb-6B. Field Emission Scanning Electron Microscopy (FESEM) (Brand Zeiss Model Auriga) was employed to study the surface morphology and pore development. Fourier transform infrared (FTIR) spectroscopy was carried out to analyse the surface functional groups of the precursor material and the prepared AC with the help of Bruker, IFS66v/S. Spectra are recorded at a range of 400 to 4000 cm−1. These analyses were performed following the method mentioned by Acharya et al.25 The size of the particles of the AC was measured by Malvern Mastersizer 2000. The instrument used laser diffraction technology based on the principle that laser scattering occurs when it hits a particle and the angle of scattering is directly related to the particle size.3. Results and discussion
3.1. Statistical analysis and model development
Statistical analysis of the data obtained for zinc adsorption and the yield of the twenty eight different samples, prepared according to the design matrix, were analysed with the aid of Design Expert software. Data for adsorption of zinc can also be better fitted to the quadratic model. The adjusted R2 value for the quadratic model for zinc adsorption is 0.8840, which is superior to the R2 value of linear, 2FI and cubic polynomial model having R2 value 0.0082, −0.2242, and 0.8435, respectively. For the yield of AC, the response was fitted to linear, two-factor interaction (2FI), quadratic and cubic polynomials to determine the most suitable model. The model fit summary showed that the quadratic model was a better fit for the data obtained as the adjusted R2 value for the quadratic model for yield of AC was 0.8889, much closer to unity than the R2 values of linear (0.5772), 2FI (0.6494) and cubic polynomials (0.8642). The respective p-values for quadratic, linear, 2FI and cubic were 0.8083, 0.2326, 0.2802, and 0.6830, that also suggests quadratic type as the most suitable model. The quadratic model equation for the yield of AC and the zinc adsorption in terms of the coded factors are given in the eqn (4) and (5):| Adsorption = 14.26 + 0.61A − 0.93B + 1.13C + 1.38D − 1.00AB − 0.18AC + 0.81AD + 0.63BC − 0.49BD − 4.98A2 − 1.58B2 − 1.70C2 | (4) |
| Yield = 57.92 − 12.83A − 4.67B − 9.04C − 7.09D + 1.71AB − 5.69AC − 3.81AD + 0.36BC − 5.0BD + 1.79CD + 5.34A2 − 7.54B2 + 4.13C2 + 12.83D2 | (5) |
ANOVA is an important tool, which tests the significance of the differences between means, thereby helps to understand the significance of a model.26 ANOVA determines the impact of the independent variables on the dependent variables in a regression analysis. As shown in Table 3, ANOVA of the regression models shows that the quadratic type to be highly significant for assessing the metal ions removal. This is evident from the Fisher's F-test where the Fmodel,adsorption = 18.82 and Fmodel,yield = 16.43, with a very low probability value of P model > F = 0.0001 for both the adsorption and yield. There was only a 0.01% chance that a model value of this magnitude could occur due to noise. In the graph of the predicted values versus actual data points, the 45 degree line should evenly split the data set. In this case, it is clear that from Fig. 1(A) and (B), the points are evenly distributed around the 45 degree line. This observation suggests that the model can closely predict the response.
| Response | Adsorption | |||||
|---|---|---|---|---|---|---|
| ANOVA for response surface reduced quadratic model | ||||||
| Source | Sum of squares | df | Mean square | F value | p-value prob. > F | |
| Model | 479.06 | 12 | 39.92 | 18.82 | <0.0001 | Significant |
| A-time | 6.66 | 1 | 6.66 | 3.14 | 0.0968 | |
| B-power | 15.72 | 1 | 15.72 | 7.41 | 0.0157 | |
| C-IR | 22.85 | 1 | 22.85 | 10.78 | 0.005 | |
| D-conc. of acid | 34.04 | 1 | 34.04 | 16.05 | 0.0011 | |
| AB | 15.97 | 1 | 15.97 | 7.53 | 0.0151 | |
| AC | 0.49 | 1 | 0.49 | 0.23 | 0.6373 | |
| AD | 10.39 | 1 | 10.39 | 4.9 | 0.0428 | |
| BC | 6.35 | 1 | 6.35 | 2.99 | 0.1042 | |
| BD | 3.9 | 1 | 3.9 | 1.84 | 0.195 | |
| A^2 | 69.74 | 1 | 69.74 | 32.88 | <0.0001 | |
| B^2 | 7.07 | 1 | 7.07 | 3.33 | 0.0879 | |
| C^2 | 8.15 | 1 | 8.15 | 3.84 | 0.0688 | |
| Residual | 31.81 | 15 | 2.12 | |||
| Lack of fit | 28.78 | 12 | 2.4 | 2.37 | 0.2589 | Not significant |
| Pure error | 3.03 | 3 | 1.01 | |||
| Cor total | 510.88 | 27 | ||||
| R2 = 0.9377, adj. R2 = 0.8879, pred. R-squared = 0.6997, adeq. precision = 14.342 | ||||||
| Response | Yield | |||||
|---|---|---|---|---|---|---|
| ANOVA for response surface quadratic model | ||||||
| Source | Sum of squares | df | Mean square | F value | p-value Prob. > F | |
| Model | 8478.74 | 14 | 605.62 | 16.43 | <0.0001 | Significant |
| A-time | 2963.47 | 1 | 2963.47 | 80.38 | <0.0001 | |
| B-power | 392.09 | 1 | 392.09 | 10.63 | 0.0062 | |
| C-IR | 1471.89 | 1 | 1471.89 | 39.92 | <0.0001 | |
| D-conc. of acid | 903.98 | 1 | 903.98 | 24.52 | 0.0003 | |
| AB | 46.58 | 1 | 46.58 | 1.26 | 0.2813 | |
| AC | 517.79 | 1 | 517.79 | 14.04 | 0.0024 | |
| AD | 231.8 | 1 | 231.8 | 6.29 | 0.0262 | |
| BC | 2.02 | 1 | 2.02 | 0.055 | 0.8187 | |
| BD | 399.6 | 1 | 399.6 | 10.84 | 0.0058 | |
| CD | 51.41 | 1 | 51.41 | 1.39 | 0.2588 | |
| A^2 | 73.58 | 1 | 73.58 | 2 | 0.1812 | |
| B^2 | 146.81 | 1 | 146.81 | 3.98 | 0.0674 | |
| C^2 | 43.91 | 1 | 43.91 | 1.19 | 0.295 | |
| D^2 | 424.65 | 1 | 424.65 | 11.52 | 0.0048 | |
| Residual | 479.3 | 13 | 36.87 | |||
| Lack of fit | 304.62 | 10 | 30.46 | 0.52 | 0.8083 | Not significant |
| Pure error | 174.67 | 3 | 58.22 | |||
| Cor total | 8958.04 | 27 | ||||
| R2 = 0.9465, adj. R2 = 0.8889, pred. R-squared 0.7671, adeq. precision = 15.133 | ||||||
Predicted R2 is an indication of the accuracy of the prediction of a response value by the model. Predicted R2 can prevent over-fitting the model and can be more useful than adjusted R2 for comparing models because it is calculated using observations not included in model estimation. A difference of 0.20 or less between adjusted R2 and predicted R2 is considered acceptable for the model to sufficiently predict the response. In the case of adsorption, the predicted R2 value was 0.6997, which was within reasonable agreement with the adjusted R2 value of 0.8879. In case of yield too, the predicted R2 was in reasonable agreement with the adjusted R2 as evident from the ANOVA table (Table 3). Adequate precision measures the signal to noise ratio, which indicates the preciseness of the model. A ratio greater than 4 is desirable. Signal to noise ratio of 14.342 in case of adsorption and 15.133 for yield of AC are considered adequate for this purpose. Thus, this model can be used to navigate in the design space. The influence of the ith observation on the fitted value in standard deviation units can be observed by the DFFITS. The DFFITS plot for zinc adsorption in Fig. 2(A) and (B) shows that only 3 data are outliers as these are outside the calculated limits. The DFFITS plot for yield in Fig. 2(B) shows that only 2 data were outliers.
3.2. Effect of multiple variables during in preparation of AC for the adsorption of Zn
The variables, namely irradiation time (T), microwave power (P), impregnation ratio (IR) and concentration of acid and the interaction between these variables determine the quality of the AC during production. ANOVA in Table 3 shows that P, IR, and concentration of acid to be very important factors as the p-value is less than 0.05.This adsorption behaviours at the lower levels and higher levels of the variables indicated that there exist optimum levels for these variables, which must be found out by optimisation of these factors.
3.3. Effect of multiple variables during the preparation of AC for yield of the AC
Yield of the product obtained from a process is an important factor considering the economic viability of the product. The selected factors T, W, IR and concentration of acid also had important effect on the yield of AC. The interaction of these variables took important role in determining the yield of the AC.Similar interaction had been observed between impregnation ratio and concentration of acid as shown in Fig. 4(D).
The general trend was the yield of AC decreased with increase in the level of the variables used. This trend was also reported by Xia et al.34 as well as Xin-hui et al.32
3.4. Optimisation of the preparation of AC
The commercial production of AC demands higher product yield for economic reasons while the adsorption efficiency is an essential product attribute for marketing. Hence it was desirable to prepare AC with optimum yield, maximising the adsorption capacity. However, it was not always possible that the AC with highest yield would have the highest adsorption capacity. In order to compromise these two values, the desirability function was applied using the Design Expert software. The process condition that had the highest desirability was selected as the optimum condition. To identify this condition, the criterion was set as “in range” for the independent variables such as T, P, IR and concentration of acid. Criterion for the responses such as adsorption and yield were set as “maximise”. Based on the highest desirability value of 0.805, the optimum process condition obtained was 11.02 min of irradiation time, 676 W of microwave power, impregnation ratio of 0.68 and undiluted sulphuric acid. The predicted adsorption was 13.73 mg g−1 and predicted yield was 77.73%. Validation experiments were conducted to confirm the values predicted from the model. In the validation experiment, it was found that the AC prepared in the optimum condition had adsorption capacity 14.6 mg g−1 and yield of AC was 72%. The error in prediction was 6.41% and 7.1%, respectively. Table 4 enlists some results obtained in recent studies for adsorption of zinc.| Adsorbent | Zinc adsorption capacity (mg g−1) | Reference |
|---|---|---|
| Physic seed hull (PSH), Jantropha curcas L | 12.29 | 35 |
| Eichhornia crassipes biomass | 12.55 | 36 |
| Activated carbon prepared from Van apple pulp | 11.72 | 37 |
| Activated carbon prepared from Phaseolus aureus hulls | 21.2 | 38 |
| Activated carbon from Hevea brasiliensis | 22.03 | 39 |
| Activated carbon from Ceiba pentandra hulls | 24.1 | 40 |
3.5. Characterisation of the AC
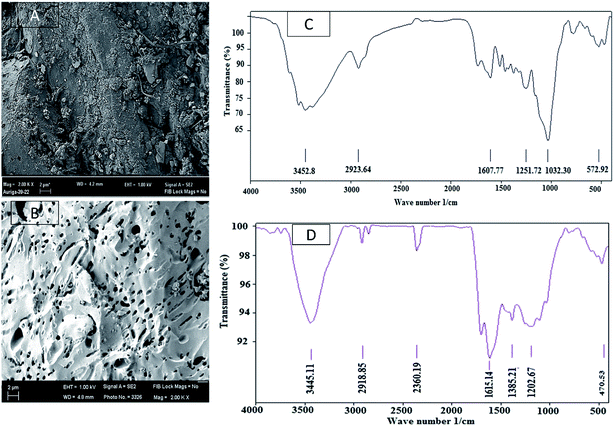
The AC was characterised by SEM, BET surface area and FTIR spectroscopy. The SEM image showed in Fig. 5(A) shows that the raw material had much less surface roughness and porosity on the surface. AC prepared in the optimum operating condition developed considerable amount of porosity after activation as evident from Fig. 5(B). The acid in the impregnation stage and microwave heating in the activation stage facilitated the formation of pores.FTIR data analysis showed that the intensity of the peak between 3500–3200 cm−1, which was attributed to O–H stretching of alcohols or phenols, decreased to a great extent. Intensity of the band of 3000–2850 cm−1 corresponding to C–H stretch of alkanes diminished in the AC. Both of these phenomena indicated that the sulphuric acid acted as dehydrating agent and had removed a considerable amount of hydrogen from the raw material41 to convert it to AC. The complex peaks around 1580–1650 cm−1 in raw palm shell that could be attributed to N–H bend of primary amines and C–C stretch of aromatic ring. The intensity of this band had also reduced. The C–O stretch for alcohols, carboxylic acids, esters, ethers corresponding to the band 1300–1000 cm−1 remained in the AC as in the raw material with a much lower intensity of the peak. This also suggested removal of hydrogen and oxygen part from the raw material.
Nitrogen adsorption/desorption isotherms determined the specific surface area through the utilisation of BET equation and was found to be 1011 m2 g−1. The pore size distribution curves showed that the AC contained mainly micro and meso pores which assisted in the adsorption process. The average pore diameter of the AC prepared at the optimum condition was 21.89 Å.
The adsorption/desorption isotherm of the AC prepared at the optimum condition in Fig. 6 shows that the curve is a “type I” isotherm, characterising micro-porous adsorbent for monolayer adsorption.42
 | ||
| Fig. 6 Nitrogen adsorption/desorption isotherm for the AC prepared at the optimum condition showing type I isotherm. | ||
4. Conclusion
The AC was prepared from PKS with sulphuric acid impregnation and by microwave heating. The heating time was only 11.02 min and this is considerably shorter than conventional production method. A statistical model was successfully adopted with the use of Design Expert software, which could predict the optimum condition efficiently. The suggested adsorption capacity and yield of AC was 13.73 mg g−1 and 77.73%, respectively, at the optimum condition of 11.02 min of irradiation time, 676 W of microwave power, impregnation ratio of 0.68 and undiluted sulphuric acid. Experimental adsorption capacity of AC was found to be 14.6 mg g−1 and yield was 72% with error in prediction of 6.41% and 7.1%, respectively. Characterisation study showed that the AC had highly porous surface and FTIR data confirmed dehydration and removal of hydrogen part from raw material to produce AC. BET surface area was found to be 1011 m2 g−1 which was considerably high.Acknowledgements
The authors are grateful to University of Malaya (Project no. UM.C/HIR/MOHE/ENG/13 and IPPP University of Malaya, project no. PG040-2012B) for providing the fund for the research work.References
- H. Marsh and F. R. Reinoso, Activated carbon, Elsevier, 2006 Search PubMed.
- A. Bhatnagar, W. Hogland, M. Marques and M. Sillanpää, Chem. Eng. J., 2013, 219, 499–511 CrossRef CAS PubMed.
- E. Saputra, S. Muhammad, H. Sun and S. Wang, RSC Adv., 2013, 3, 21905–21910 RSC.
- V. O. Njoku, K. Y. Foo, M. Asif and B. H. Hameed, Chem. Eng. J., 2014, 250, 198–204 CrossRef CAS PubMed.
- J. M. V. Nabais, C. E. C. Laginhas, P. J. M. Carrott and M. M. L. Ribeiro Carrott, Fuel Process. Technol., 2011, 92, 234–240 CrossRef CAS PubMed.
- R. Baccar, J. Bouzid, M. Feki and A. Montiel, J. Hazard. Mater., 2009, 162, 1522–1529 CrossRef CAS PubMed.
- M. U. Dural, L. Cavas, S. K. Papageorgiou and F. K. Katsaros, Chem. Eng. J., 2011, 168, 77–85 CrossRef CAS PubMed.
- G. San Miguel, G. D. Fowler and C. J. Sollars, Carbon, 2003, 41, 1009–1016 CrossRef CAS.
- T. Tay, S. Ucar and S. Karagöz, J. Hazard. Mater., 2009, 165, 481–485 CrossRef CAS PubMed.
- P.-T. Yeung, P.-Y. Chung, H.-C. Tsang, J. Cheuk-On Tang, G. Yin-Ming Cheng, R. Gambari, C.-H. Chui and K.-H. Lam, RSC Adv., 2014, 4, 38839–38847 RSC.
- A. D. Russell, E. I. Antreou, S. S. Lam, C. Ludlow-Palafox and H. A. Chase, RSC Adv., 2012, 2, 6756–6760 RSC.
- E. T. Thostenson and T. W. Chou, Composites, Part A, 1999, 30, 1055–1071 CrossRef.
- M. A. Ahmad and R. Alrozi, Chem. Eng. J., 2010, 165, 883–890 CrossRef CAS PubMed.
- NIST/SEMATECH, in Process Improvement, ed. P. Tobias and L. Trutna, 2012, vol. 2013 Search PubMed.
- R. Verseput, Selecting the right central composite design for response surface methodology applications, 22 July 2014, http://www.qualitydigest.com/june01/html/doe.html.
- M. Arulkumar, K. Thirumalai, P. Sathishkumar and T. Palvannan, Chem. Eng. J., 2012, 185–186, 178–186 CrossRef CAS PubMed.
- B. S. Kaith, R. Sharma, S. Kalia and M. S. Bhatti, RSC Adv., 2014, 4, 40339–40344 RSC.
- R. J. E. Martins, R. Pardo and R. A. R. Boaventura, Water Res., 2004, 38, 693–699 CrossRef CAS PubMed.
- C. Lu, H. Chiu and C. Liu, Ind. Eng. Chem. Res., 2006, 45, 2850–2855 CrossRef CAS.
- V. K. Gupta and S. Sharma, Ind. Eng. Chem. Res., 2003, 42, 6619–6624 CrossRef CAS.
- F. Abnisa, W. M. A. Wan Daud and J. N. Sahu, Biomass Bioenergy, 2011, 35, 3604–3616 CrossRef CAS PubMed.
- F. Karacan, U. Ozden and S. Karacan, Appl. Therm. Eng., 2007, 27, 1212–1218 CrossRef CAS PubMed.
- E. Bayraktar, Process Biochem., 2001, 37, 169–175 CrossRef CAS.
- D. C. Montgomery, D. C. Montgomery and D. C. Montgomery, Design and analysis of experiments, Wiley, New York, 1984 Search PubMed.
- J. Acharya, J. N. Sahu, C. R. Mohanty and B. C. Meikap, Chem. Eng. J., 2009, 149, 249–262 CrossRef CAS PubMed.
- R. Sen and T. Swaminathan, Biochem. Eng. J., 2004, 21, 141–148 CrossRef CAS PubMed.
- H. Deng, G. Zhang, X. Xu, G. Tao and J. Dai, J. Hazard. Mater., 2010, 182, 217–224 CrossRef CAS PubMed.
- L.-Y. Hsu and H. Teng, Fuel Process. Technol., 2000, 64, 155–166 CrossRef CAS.
- M. Jagtoyen and F. Derbyshire, Carbon, 1998, 36, 1085–1097 CrossRef CAS.
- A.-N. A. El-Hendawy, A. J. Alexander, R. J. Andrews and G. Forrest, J. Anal. Appl. Pyrolysis, 2008, 82, 272–278 CrossRef CAS PubMed.
- R. Hoseinzadeh Hesas, A. Arami-Niya, W. M. A. Wan Daud and J. N. Sahu, Chem. Eng. Res. Des., 2013, 91(12), 2447–2456 CrossRef CAS PubMed.
- D. Xin-hui, C. Srinivasakannan, P. Jin-hui, Z. Li-bo and Z. Zheng-yong, Fuel Process. Technol., 2011, 92, 394–400 CrossRef PubMed.
- K. Y. Foo and B. H. Hameed, Bioresour. Technol., 2012, 103, 398–404 CrossRef CAS PubMed.
- H.-y. Xia, J.-h. Peng, L.-b. Zhang, X.-y. MA, J.-h. TU and S.-m. Zhang, Chemical Engineering, 2007, 1, 012 Search PubMed.
- M. Mohammad, S. Maitra, N. Ahmad, A. Bustam, T. K. Sen and B. K. Dutta, J. Hazard. Mater., 2010, 179, 363–372 CrossRef CAS PubMed.
- C. Mahamadi and T. Nharingo, Bioresour. Technol., 2010, 101, 859–864 CrossRef CAS PubMed.
- T. Depci, A. R. Kul and Y. Önal, Chem. Eng. J., 2012, 200–202, 224–236 CrossRef CAS PubMed.
- M. M. Rao, D. K. Ramana, K. Seshaiah, M. C. Wang and S. W. C. Chien, J. Hazard. Mater., 2009, 166, 1006–1013 CrossRef CAS PubMed.
- H. Kalavathy, B. Karthik and L. R. Miranda, Colloids Surf., B, 2010, 78, 291–302 CrossRef CAS PubMed.
- M. M. Rao, G. P. C. Rao, K. Seshaiah, N. V. Choudary and M. C. Wang, Waste Manag., 2008, 28, 849–858 CrossRef CAS PubMed.
- R. H. Hesas, A. Arami-Niya, W. M. A. W. Daud and J. Sahu, BioResources, 2013, 8, 2950–2966 Search PubMed.
- O. Carmody, R. Frost, Y. Xi and S. Kokot, Surf. Sci., 2007, 601, 2066–2076 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |