A facile route for the synthesis of mesoporous melamine-formaldehyde resins for hexavalent chromium removal†
Zhongfei Lv,
Changsheng Liang,
Jiayang Cui,
Yanan Zhang and
Shiai Xu*
Shanghai Key Laboratory of Advanced Polymeric Material, Key Laboratory for Ultrafine Materials of Ministry of Education, School of Materials Science and Engineering, East China University of Science and Technology, Shanghai 200237, China. E-mail: saxu@ecust.edu.cn; Fax: +86 021 6425 3775; Tel: +86 021 6425 3353
First published on 3rd February 2015
Abstract
A highly mesoporous melamine-formaldehyde resin (MMF) has been successfully synthesized through a facile route. It shows a relatively high surface area (317 m2 g−1), uniform pore sizes (8.0 nm), enhanced adsorption capacity (66.65 mg g−1) and selectivity for hexavalent chromium.
Hexavalent chromium (Cr(VI)) is a highly toxic metallic element widely present in the industrial waste water from a variety of industrial processes, such as metal finishing, dyes, inks, pigments, ceramics and leather tanning.1,2 Cr(VI) can easily penetrate cell walls, damaging cell structure and resulting in various cancers.3–5 Thus, removal of Cr(VI) from industrial waste water is of great public health importance. There are many methods available for this purpose, including sulfide precipitation, ion exchange, solvent extraction and adsorption. Among these methods, adsorption seems to be the most simple and effective.6–10 For example, activated carbon (AC) is often used as an adsorbent for the removal of Cr(VI) due to its high surface area and abundant micropores.11,12 However, further treatment is often needed to enhance its adsorption capacity and selectivity, such as oxidation, acidation, alkalization and impregnation of foreign materials, thus increasing the cost and limiting its applications.13–21 Therefore, many other adsorbents have also been synthesized, such as melamine-formaldehyde resins,22,23 chitosans24 and their chemically modified materials.25–29 These adsorbents have more functional groups and can increase the adsorption sites for Cr(VI).
In 1992, an ordered mesoporous material (OMM), MCM-41 was successfully synthesized from the liquid-crystal templates.30 Over the past decades, some other OMMs have been reported and attracted great interest for their special pore structure, large surface area, uniform pore sizes and wide applications in the catalysis, adsorption, separation, and biomedicine.31–39 However, for organic mesoporous materials, it seemed that such organic templates are not good for compatibility between the template and the polymer precursor, which should be carefully adjusted so that mesophase formation can occur. And so far, the organic–organic self-assemblies applied for the synthesis of mesoporous resins are mainly phenolic based polymers.35 Besides, many other techniques such as templating method (hard-template and soft-template),40,41 ionothermal method,42 dynamic polymerization43 or based on Schiff base chemistry method44 were used to prepare the porous polymers and made some achievements in the application of energy45 and catalysis.46
In this study, we report a facile route to synthesize mesoporous melamine-formaldehyde resins (MMF) from a mixture of copolymer (F127), melamine and paraformaldehyde in aqueous solution via a self-assembly method (Scheme S1 and Fig. S1†). Compared with the other synthetic methods for MMF, our approach is low cost (using melamine and paraformaldehyde rather than special monomer hexamethoxymethyl melamine),47 easy to synthesis (hydrothermally synthesized in an autoclave without using any inorganic templates)48 and less environment pollution (without using supervirulent organic solvent or carcinogenic formaldehyde).49–51 The resulting MMF is proven to be an efficient adsorbent for Cr(VI), and has better adsorption capacity and selectivity than conventional AC. More importantly, temperature has little impact on the adsorption capacity of MMF for Cr(VI), indicating that this adsorbent is applicable in any season.
The N2 sorption isotherms, SEM and TEM images of MMF sample are showed in Fig. 1. The N2 sorption isotherms of MMF (Fig. 1A) exhibit a typical type-IV curve with a hysteresis loop at 0.44–0.90, confirming the presence of mesoporosity in MMF.52 The pore size calculated using the Barrett–Joyner–Halenda (BJH) method centers at 8.0 nm (Fig. 1B), and the BET surface area is at 317 m2 g−1 and pore volume is at 0.58 cm3 g−1 (Table S1†). The SEM image (Fig. 1C) shows that MMF is highly mesoporous with a few larger pores. In addition, the TEM image (Fig. 1D) clearly reveals the uniform pore size, which is well agreement with pore size distributions. Furthermore, MMF is rich in inherent aminal (–NH–CH2–NH–) and triazine groups (Fig. S2 and S3†), which could act as the activated sites for catching toxic Cr(VI) from water.51 More interestingly, there is only methylene linkage but no methylene–ether linkage in the sample, which is very important for the stability of the polymer networks.53,54 Thus, the TGA profile shows that MMF is stable up to ∼300 °C (Fig. S4†). In addition, the elemental analysis of MMF shows that the molar ratio of C/H/N/O is 1.00/1.82/1.13/0.40 (Table S2†).
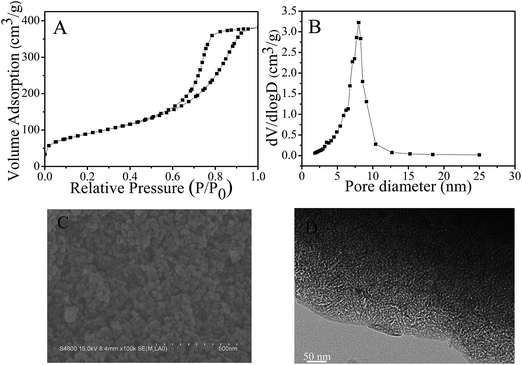 | ||
| Fig. 1 (A) N2 sorption isotherms, (B) pore size distributions, (C) SEM image and (D) TEM image of MMF samples. | ||
Batch experiments were carried out to evaluate the adsorption capacity and selectivity of MMF and AC for Cr(VI). It is evident in Fig. 2 and Table S3† that MMF has higher adsorption capacity (35.45 mg g−1) and better selectivity for Cr(VI), but lower adsorption capacity for some other ions, such as Fe(III), Cu(II) and Ni(II). In contrast, although the commercial adsorbent AC has higher surface area (1435 m2 g−1) and porosity, it has relatively lower adsorption capacity and selectivity for Cr(VI). Moreover, the other mesoporous melamine-formaldehyde resins such as MMF-1 and MMF-2 (N2 sorption isotherms and pore size distributions of MMF-1 and MMF-2 were showed in Fig. S5†) show relatively lower or similar adsorption property with the resulting MMF.
 | ||
| Fig. 2 Adsorption of Cr(VI), Cr(III), Fe(III), Cu(II) and Ni(II) by MMF and AC (0.05 g of MMF or AC was added to 50 ml of 40.0 ppm metal ions solutions and stirred at 150 rpm at 20 °C for 20 min). | ||
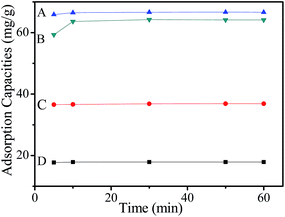
Fig. 3 and Table S4† show the adsorption capacity of MMF for Cr(VI) as a function of contact time with different initial concentrations. The adsorption rate of metal uptake is very rapid and the maximum uptake is reached within 5 min, after which there is no evident increase in adsorption. These results indicate that the adsorption of Cr(VI) by MMF takes place rapidly, thus saving time and costs for waste water treatment. However, the adsorption rate of Cr(VI) by other adsorbents shows a wide range of adsorption times.55 In addition, the adsorption isotherms are a function of the initial Cr(VI) concentration, but the concentration-dependent adsorption only works to a certain extent. As shown in Fig. 3, the adsorption capacity of MMF increases rapidly from 19.0 ppm to 79.1 ppm and obtains the maximum adsorption capacity (66.65 mg g−1) at the initial Cr(VI) concentration of 142.6 ppm.
Adsorption experiments were performed at 20, 25, 30, 35 and 40 °C to investigate the effect of temperature on the adsorption of Cr(VI) by MMF (Fig. 4 and Table S5†) show that the adsorption of Cr(VI) ions increases slightly with increment of temperature from 20 to 30 °C, and then decreases with further increase of temperature from 30 to 40 °C. The adsorption capacity of MMF reaches the maximum of 37.55 mg g−1 at 30 °C. However, it needs to be pointed out that the overall change in the adsorption of Cr(VI) with the temperature is fairly subtle, indicating that MMF could be widely used for the removal of Cr(VI) without seasonal restrictions.
 | ||
| Fig. 4 Effect of temperature on the adsorption of Cr(VI) by MMF (0.05 g of MMF was added to 50 ml of 40.7 ppm Cr(VI) solutions, and stirred at 150 rpm at pH 6.0 for 20 min). | ||
The effect of pH on the adsorption of Cr(VI) by MMF is shown in Fig. 5 and Table S6.† The maximum adsorption of Cr(VI) is observed at pH 6.0. The pH dependence of metal adsorption has been shown to be largely related to the type and ionic state of the functional groups present in the adsorbent and the metal chemistry in the solution. The distributions of various forms of Cr(VI) ions in the aqueous solution were determined using appropriate chemical reaction equilibrium analyses. Cr anions are known to exist in the following forms depending on the pH of the aqueous solution.56
H2CrO4 ↔ H+ + HCrO4− log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K1 = −0.8) K1 = −0.8)
| (1) |
HCrO4− ↔ H+ + CrO42− (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K2 = −6.5) K2 = −6.5)
| (2) |
2HCrO4− ↔ H2O + Cr2O72− (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K3 = 1.52) K3 = 1.52)
| (3) |
HCr2O7− ↔ H+ + Cr2O72− log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K4 = 0.07) K4 = 0.07)
| (4) |
HCrO4− and CrO42− are the predominant species in total chromium concentration. HCrO4− is the predominant at pH 2–6.5, whereas CrO42− is predominant at pH > 6.5.57 Under a lower pH, the surface of MMF becomes highly protonated and would not bind with metal ions. As pH increases, the amine groups of MMF become non-protonated and could act as coordinating ligands for metal ions. However, if pH continues to increase, the surface will be negatively charged, greatly weakening the electrostatic attraction between the sorbent and negatively charged Cr(VI) anions, thus reducing the removal efficiency. Moreover, there is a competition between OH− and Cr ions, especially at a high pH. More interestingly, the effect of pH on the adsorption of Cr(VI) indicates that the Cr(VI) adsorbed by MMF could be recovered, and the sorbent could be reused. In this study, Cr/MMF (MMFadsorbed Cr) was subjected to basic treatment to recover the adsorbed Cr(VI) ions. Table S7† shows the desorption percent of Cr/MMF at different times. It shows that the use of 0.1 M NaOH solution allows a ∼100% recovery of the adsorbed Cr(VI). After basic treatment, MMF shows relatively higher adsorption capacity for Cr(VI) again (Table S8†).
The XPS spectra (Fig. S6 and S7†) of MMF before and after the adsorption of Cr show that the binding energy of N1s and O1s decrease in the Cr/MMF (Fig. S7†). More importantly, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups of MMF at binding energy of 287.6 eV become stronger, which is possibly attributed to the enhancement of the chelating ligands between the groups of Cr(VI), C, N and O. Thus, there may be some interactions between C, N and O with Cr(VI), which could explain the excellent adsorption capacity and selectivity of MMF.58–62 Theses results indicate that the presence of the surface functional groups rather than surface area or pore volumes, contributes to increasing the adsorption and selectivity of MMF for Cr(VI).
N groups of MMF at binding energy of 287.6 eV become stronger, which is possibly attributed to the enhancement of the chelating ligands between the groups of Cr(VI), C, N and O. Thus, there may be some interactions between C, N and O with Cr(VI), which could explain the excellent adsorption capacity and selectivity of MMF.58–62 Theses results indicate that the presence of the surface functional groups rather than surface area or pore volumes, contributes to increasing the adsorption and selectivity of MMF for Cr(VI).
Conclusions
In this study, we reported a facile route to synthesize MMF, which could quickly and efficiently remove toxic Cr(VI) from water within 5 minutes. Such fast adsorption rate and high adsorption capacity of MMF is mainly attributed to its abundant functional groups, such as high densities of amine and triazine groups. More importantly, the adsorbed Cr(VI) could be recovered and the MMF is easily regenerated by basic treatment. The MMF is easy to synthesis, low cost and environment-friendly, especially, it can remove the toxic Cr(VI) efficiently and quickly, these advantages make it to be an attractive adsorbent for the purification of waste and contaminated water.Acknowledgements
This work is supported by the National Natural Science Foundation of China (no. 51463020) and the Fundamental Research Funds for the Central Universities (WD1315012).Notes and references
- D. Mohan and C. U. Pittman Jr, J. Hazard. Mater., 2006, B137, 762 CrossRef PubMed.
- M. Owlad, M. K. Aroua, W. A. W. Daud and S. Baroutian, Water, Air, Soil Pollut., 2009, 200, 59 CrossRef CAS.
- C. Barnowski, N. Jakubowski, D. Stuewer and J. A. C. Broekaert, J. Anal. At. Spectrom., 1997, 12, 1155 RSC.
- A. M. Zayed and N. Terry, Plant Soil, 2003, 249, 139 CrossRef CAS.
- R. A. Gil, S. Cerutti, J. A. Gásquez, R. A. Olsina and L. D. Martinez, Talanta, 2006, 68, 1065 CrossRef CAS PubMed.
- R. F. D. Costa, M. A. S. Rodrigues and J. Z. Ferreria, Sep. Sci. Technol., 1998, 33, 1135 CrossRef.
- S. Rengaraj, K.-H. Yeon and S.-H. Moon, J. Hazard. Mater., 2001, B87, 273 CrossRef.
- Y. Nakano, K. Takeshita and T. Tsutsumi, Water Res., 2001, 35, 496 CrossRef CAS.
- T. N. de Castro Dantas, A. A. Dantas Neto and M. C. P. D. A. Moura, Water Res., 2001, 35, 2219 CrossRef CAS.
- F. Fu and Q. Wang, J. Environ. Manage., 2011, 92, 407 CrossRef CAS PubMed.
- C. P. Huang and M. H. Wu, Water Res., 1977, 11, 673 CrossRef CAS.
- M. Pérez-Candela, J. M. Martín-Martínez and R. Torregrosa-Maciá, Water Res., 1995, 29, 2174 CrossRef.
- A. Ahmadpour and D. D. Do, Carbon, 1996, 34, 471 CrossRef CAS.
- C. Toles, S. Rimmer and J. C. Hower, Carbon, 1996, 34, 1419 CrossRef CAS.
- J. Guo and A. C. Lua, Microporous Mesoporous Mater., 1999, 32, 111 CrossRef CAS.
- D. Aggarwal, M. Goyal and R. C. Bansal, Carbon, 1999, 37, 1989 CrossRef CAS.
- F. Adib, A. Bagreev and T. J. Bandosz, Langmuir, 2000, 16, 1980 CrossRef CAS.
- K. Selvi, S. Pattabhi and K. Kadirvelu, Bioresour. Technol., 2001, 80, 87 CrossRef CAS.
- L. Khezami and R. Capart, J. Hazard. Mater., 2005, B123, 223 CrossRef PubMed.
- M. Valix, W. H. Cheung and K. Zhang, J. Hazard. Mater., 2006, B135, 395 CrossRef PubMed.
- S. X. Liu, X. Chen, X. Y. Chen, Z. F. Liu and H. L. Wang, J. Hazard. Mater., 2007, 141, 315 CrossRef CAS PubMed.
- B. Demirata, I. Tor, H. Filik and H. Afsar, Fresenius. J. Anal. Chem., 1996, 356, 375 CrossRef CAS PubMed.
- B. Demirata, Microchim. Acta, 2001, 136, 143 CrossRef CAS.
- T. N. de Castro Dantas, A. A. Dantas Neto, M. C. P. d. A. Moura, E. L. Barros Neto and E. de Paiva Telemaco, Langmuir, 2001, 17, 4256 CrossRef.
- N. Sankararamakrishnan, A. Dixit, L. Iyengar and R. Sanghi, Bioresour. Technol., 2006, 97, 2377 CrossRef CAS PubMed.
- A. Ramesh, H. Hasegawa, W. Sugimoto, T. Maki and K. Ueda, Bioresour. Technol., 2008, 99, 3801 CrossRef CAS PubMed.
- P. Baroni, R. S. Vieira, E. Meneghetti, M. G. C. da Silva and M. M. Beppu, J. Hazard. Mater., 2008, 152, 1155 CrossRef CAS PubMed.
- N. G. Kandile and A. S. Nasr, Carbohydr. Polym., 2009, 78, 753 CrossRef CAS PubMed.
- X.-J. Hu, J.-S. Wang, Y.-G. Liu, X. Li, G.-M. Zeng, Z.-L. Bao, X.-X. Zeng, A.-W. Chen and F. Long, J. Hazard. Mater., 2011, 185, 306 CrossRef CAS PubMed.
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710 CrossRef CAS.
- P. T. Tanev, M. Chibwe and T. J. Pinnavaia, Nature, 1994, 368, 321 CrossRef CAS PubMed.
- P. Yang, D. Zhao, D. I. Margolese, B. F. Chmelka and G. D. Stucky, Nature, 1998, 396, 152 CrossRef CAS PubMed.
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stuck, Science, 1998, 279, 548 CrossRef CAS.
- R. Ryoo, S. H. Joo, M. Kruk and M. Jaroniec, Adv. Mater., 2001, 13, 677 CrossRef CAS.
- Y. Meng, D. Gu, F. Q. Zhang, Y. F. Shi, H. F. Yang, Z. Li, C. Z. Yu, B. Tu and D. Y. Zhao, Angew. Chem., Int. Ed., 2005, 44, 7053 CrossRef CAS PubMed.
- A. Corma, Chem. Rev., 1997, 97, 2373 CrossRef CAS PubMed.
- A. M. Liu, K. Hidajat, S. Kawi and D. Y. Zhao, Chem. Commun., 2000, 1145 RSC.
- J. C. Groen, L. A. A. Peffer and J. Pérez-Ramírez, Microporous Mesoporous Mater., 2003, 60, 1 CrossRef CAS.
- M. Vallet-Regí, F. Balas and D. Arcos, Angew. Chem., Int. Ed., 2007, 46, 7548 CrossRef PubMed.
- A. Thomas, F. Goettmann and M. Antonietti, Chem. Mater., 2008, 20, 738 CrossRef CAS.
- H.-P. Hentze and M. Antonietti, Curr. Opin. Solid State Mater. Sci., 2001, 5, 343 CrossRef CAS.
- P. Kuhn, M. Antonietti and A. Thomas, Angew. Chem., Int. Ed., 2008, 47, 3450 CrossRef CAS PubMed.
- P. Kuhn, A. Thomas and M. Antonietti, Macromolecules, 2009, 42, 319 CrossRef CAS.
- M. G. Schwab, B. Fassbender, H. W. Spiess, A. Thomas, X. Feng and K. Müllen, J. Am. Chem. Soc., 2009, 131, 7216 CrossRef CAS PubMed.
- A. Thomas, P. Kuhn, J. Weber, M.-M. Titirici and M. Antonietti, Macromol. Rapid Commun., 2009, 30, 221 CrossRef CAS PubMed.
- A. Thomas, A. Fischer, F. Goettmann, M. Antonietti, J.-O. Müller, R. Schlögl and J. M. Carlsson, J. Mater. Chem., 2008, 18, 4893 RSC.
- K. Kailasam, Y.-S. Jun, P. Katekomol, J. D. Epping, W. H. Hong and A. Thomas, Chem. Mater., 2010, 22, 428 CrossRef CAS.
- A. Derylo-Marczewska and J. Goworek, Langmuir, 2001, 17, 6518 CrossRef CAS.
- M. X. Tan, L. Gu, N. Li, J. Y. Ying and Y. G. Zhang, Green Chem., 2013, 15, 1127 RSC.
- M. X. Tan, Y. Zhang and J. Y. Ying, ChemSusChem, 2013, 6, 1186 CrossRef CAS PubMed.
- M. X. Tan, Y. N. Sum, J. Y. Ying and Y. Zhang, Energy Environ. Sci., 2013, 6, 3254 CAS.
- M. Kruk and M. Jaroniec, Chem. Mater., 2003, 15, 2942 CrossRef CAS.
- Z. Lv, Q. Sun, X. Meng and F.-S. Xiao, J. Mater. Chem. A, 2013, 1, 8630 CAS.
- B. Friedel and S. Greulich-Weber, Small, 2006, 2, 859 CrossRef CAS PubMed.
- V. K. Gupta, S. Agarwal and T. A. Saleh, Water Res., 2011, 45, 2207 CrossRef CAS PubMed.
- A. K. Sengupta and D. Cllfford, Environ. Sci. Technol., 1986, 20, 149 CrossRef CAS PubMed.
- H.-J. Park and L. L. Tavlarides, Ind. Eng. Chem. Res., 2008, 47, 3401 CrossRef CAS.
- A. Derylo-Marczewska, J. Goworek, S. Pikus and E. Kobylas, Langmuir, 2002, 18, 7538 CrossRef CAS.
- L. Dupont and E. Guillon, Environ. Sci. Technol., 2003, 37, 4235 CrossRef CAS.
- H. Jabeen, V. Chandra, S. Jung, J. W. Lee, K. S. Kim and S. B. Kim, Nanoscale, 2011, 3, 3583 RSC.
- M. Barber, J. A. Connor, M. F. Guest, M. B. Hall, I. H. Hillier and W. N. E. Meredith, Faraday Discuss. Chem. Soc., 1972, 54, 219 RSC.
- S. Pignataro and A. Foffani, Chem. Phys. Lett., 1973, 20, 350 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra16866f |
| This journal is © The Royal Society of Chemistry 2015 |