Porous hexagonal cobalt oxyhydroxide sheets with attached nickel hydroxide nanoparticles as electrode materials for electrochemical supercapacitors
Mao-Sung Wu*,
Chun-Yi Tsai and
Yu-Sheng Lai
Department of Chemical and Materials Engineering, National Kaohsiung University of Applied Sciences, Kaohsiung 807, Taiwan. E-mail: ms_wu@url.com.tw; Fax: +886-7-3830674
First published on 27th January 2015
Abstract
Ni(OH)2 nanoparticles were self-assembled on porous hexagonal CoOOH sheets by a simple cathodic electrophoresis in CoOOH suspension with nickel nitrate additive. A significant shift of redox peaks in the Ni(OH)2/CoOOH composite electrodes compared with the bare Ni(OH)2 electrode indicated the formation of mixed Co–Ni hydroxide layers. The amount of Ni(OH)2 nanoparticles on the porous CoOOH sheet dominated the capacitive behavior of the composite electrodes. The Ni(OH)2/CoOOH electrode with 20 wt% of Ni(OH)2 prepared in 1 mM nickel nitrate showed the highest specific capacitance and good cycle-life stability, its specific capacitance discharged at 1 A g−1 could reach as high as 1537 F g−1 much higher than that of bare CoOOH (52 F g−1) and Ni(OH)2 (785 F g−1) electrodes. A noticeable improvement in capacitive behavior resulted from the porous conductive CoOOH sheets that facilitated the transport of electron and electrolyte.
Introduction
Electrochemical supercapacitors bridge the gap between electric double-layer capacitors (EDLCs) and the traditional rechargeable batteries in terms of the energy density and power density, opening exciting opportunities for numerous applications in portable power.1 Several materials such as carbon materials, metal oxides, and metal sulfides have been characterized as promising electrode materials for application in supercapacitors.2–11 Among them, transition metal oxides have gained much attention because of their high specific capacitance, easy preparation, and low raw material cost. Nickel- and cobalt-based oxides/hydroxides are more suitable for application in asymmetric supercapacitors with alkaline electrolyte due to their high redox capacitance and good corrosion resistance to the alkaline electrolyte.12,13Due to the poor electrical conductivity of bare nickel hydroxides, permanently conductive additives such as cobalt oxide, carbon black, and nickel powder are added to the nickel hydroxide electrodes to improve the electrical conductivity of the bulk electrodes.14–17 Among these additives, the most significant breakthrough is the addition of cobalt oxide/hydroxide to increase the electrical conductivity of the nickel hydroxide electrodes. The cobalt oxide additives have also demonstrated a number of advantages, such as restrained electrode expansion during cycles and increasing the charge efficiency at elevated temperature.15–17 The increase of utilization and electrical conductivity is primarily due to the formation of a conductive thin CoOOH film on the surface of the porous nickel hydroxide electrode as a conductive network for the fast transport of electrons.17 Partial substitution of Ni by Co, Al, Zn, or Mn may considerably improve the capacitive behavior of the nickel hydroxide electrodes due to the formation of layered double hydroxide (LDH).18–29 Among these LDH materials, Co–Ni hydroxides have recently become a focus of interest. Ni and Co are co-incorporated in the host layer, thus an improved capacity and cycling stability can be largely achieved as compared with the bare nickel hydroxides.30,31
Coating nickel hydroxides on three-dimensional (3D) conductive scaffolds like Ni foam, Ni wires, carbon fabric, and carbon nanotube arrays could effectively enhance their capacitive behavior due to the unique 3D structure that favors the transport of ions and electrons.32–39 Following these ideas, one could infer that a similar effect can be achieved by providing an electrically conductive layer of CoOOH for supporting the nickel hydroxide nanostructures. The similarities between Ni and Co offer an opportunity to yield hybrid materials with enhanced capacitive behavior. The nano-sized nickel oxide/hydroxide and cobalt oxide/hydroxide is benefit to the diffusion of OH− ions due to the shortened diffusion path in solid phase.36,40–47 In this work, the nickel hydroxide nanoparticles were electrophoretically self-assembled on the porous conductive CoOOH nanosheets for high-performance supercapacitors. Significantly, electrochemical characterizations reveal that the unique structure of porous conductive CoOOH nanosheets with attached nickel hydroxide nanoparticles exhibits a high specific capacitance and excellent cycling stability. Thus, the proposed strategy may offer an effective way to obtain the high-performance hybrid electrodes for potential application in the electrochemical supercapacitors.
Experimental
Hexagonal cobalt oxyhydroxide sheets were synthesized by a simple chemical precipitation. In a typical procedure, 500 mL of 1 M KOH solution was instilled into 200 mL of 0.13 M Co(NO3)2·6H2O solution under continuous magnetic stirring (120 rpm) at room temperature for 24 h. The resultant precipitate was rinsed by de-ionized water using ultrasonic cleaner several times until the solution became neutral. The slurry was centrifuged, and the supernatant was decanted away. The resultant slurry consisted of about 10 wt% cobalt oxyhydroxide and 90 wt% water. The hexagonal cobalt oxyhydroxide sheet with attached nickel hydroxide nanoparticles was carried out by electrophoretic deposition (EPD) technique. Type-304 stainless steel (SS) sheet was used as the electrode substrate. SS sheet was cut into pieces of size 2 cm × 2 cm. The tailored SS sheets were cleaned by rinsing with acetone then washed with de-ionized water to remove any trace of contaminants from their surface. Cathodic EPD of electrodes was carried out by applying a potential difference of −60 V (Keithley, 2400 source meter) across the working (negative electrode, SS sheet) and counter (positive electrode, Pt sheet, 2 cm × 2 cm) electrodes at room temperature. EPD bath was prepared by suspending cobalt oxyhydroxide slurry (0.2 g) in isopropyl alcohol (IPA, 50 mL) using ultrasonic cleaner for 10 min to form a homogeneous suspension. A small amount of Ni(NO3)2·6H2O was added into the EPD bath as a charging agent, and the bath was stored at room temperature in screw-cap bottle for 12 h before EPD. The concentration of nickel nitrate additive in the bath was varied in the range of 0–2 mM. Bare nickel hydroxide electrode was prepared in the same way as the cobalt oxyhydroxide-supported nickel hydroxide electrode but without addition of cobalt oxyhydroxide slurry. The working electrode was positioned face to face between two counter electrodes and distance between working and counter electrodes was held approximately at 1 cm. After EPD, the working electrodes were rinsed with de-ionized water and then dried at 150 °C for 1 h in air.The internal microstructure of cobalt oxyhydroxides and their composites was characterized by a transmission electron microscopy (TEM, Jeol JEM-1400). For the TEM observation, the specimen was scraped off from the SS substrate and suspended in ethanol using ultrasonic cleaner for 5 min. Zeta potential analyzer (Brookhaven, 90Plus) was used to measure the zeta potential of the cobalt oxyhydroxide suspension. The crystal structure of cobalt oxyhydroxides and their composites was identified by an X-ray diffractometer (Bruker, D8 Advance) with a Cu Kα target (average wavelength = 1.54056 Å). A field-emission scanning electron microscope (FE-SEM, Auriga) was used to examine the surface morphology of deposited electrodes. The elemental analysis was carried out by use of an energy dispersive spectrometer (EDS, Auriga) coupled with the FE-SEM. Capacitive behavior of the cobalt oxyhydroxide electrodes was determined by the cyclic voltammetry in a homemade four-electrode cell equipped with a working electrode, two counter electrodes (Pt, 2 cm × 2 cm), and saturated Ag/AgCl electrode (reference electrode) in 1 M KOH solution at room temperature. The working electrode was placed in between two counter electrodes. The potential was linearly swept with time at a scan rate of 1 mV s−1 using a potentiostat/galvanostat (CH Instruments, CHI 608) in the potential range of 0.0–0.45 V. Galvanostatic charge and discharge tests were carried out by a source meter (Keithley, 2400) at various current densities in the potential range of 0.0–0.45 V. Electrochemical impedance was performed by CHI 608 under an open-circuit condition (about 0.33 V). A small ac perturbation amplitude of 10 mV versus the open-circuit potential was applied in a frequency range from 50 kHz down to 0.1 Hz. Cycle-life stability of the cobalt oxyhydroxide electrodes was investigated using a source meter (Keithley, 2400) at a charge/discharge current density of 10 A g−1.
Results and discussion
Fig. 1 shows the XRD patterns of cobalt oxyhydroxide samples with and without attached nickel hydroxides. All characteristic diffraction peaks of bare cobalt oxyhydroxide sample prepared in the absence of nickel nitrate additive could be assigned to the CoOOH (JCPDS no. 73-1213) with a space group of P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (166). No other diffraction peak is observed, indicating the high purity of cobalt oxyhydroxide. The cobalt ions react with hydroxide ions to form cobalt hydroxide during precipitation reaction in alkaline media. The cobalt hydroxide (Co2+) may convert into cobalt oxyhydroxide (Co3+) because of the well-known stability of Co3+ in KOH solution.40,48 The zeta potential of the cobalt oxyhydroxide suspension in IPA–water solution was measured to be approximately 27 mV. Thus, the cobalt oxyhydroxide particles without nickel nitrate additive could be readily deposited on the SS substrate by cathodic EPD. The cobalt oxyhydroxide particles with positively charged surface could move towards negative electrode (SS) and subsequently deposit on the electrode under an applied electric field during cathodic EPD. The cobalt oxyhydroxide with attached nickel hydroxides could be carried out by the cathodic EPD in the presence of nickel nitrate additive in IPA–water solution. The cathodic deposition of nickel hydroxide results from the chemical precipitation reaction between nickel ions and hydroxide ions. The generation of hydroxide ions is accompanied with the cathodic EPD resulting from the electrochemical reduction of nitrate ions and water molecules through the following reactions:42
m (166). No other diffraction peak is observed, indicating the high purity of cobalt oxyhydroxide. The cobalt ions react with hydroxide ions to form cobalt hydroxide during precipitation reaction in alkaline media. The cobalt hydroxide (Co2+) may convert into cobalt oxyhydroxide (Co3+) because of the well-known stability of Co3+ in KOH solution.40,48 The zeta potential of the cobalt oxyhydroxide suspension in IPA–water solution was measured to be approximately 27 mV. Thus, the cobalt oxyhydroxide particles without nickel nitrate additive could be readily deposited on the SS substrate by cathodic EPD. The cobalt oxyhydroxide particles with positively charged surface could move towards negative electrode (SS) and subsequently deposit on the electrode under an applied electric field during cathodic EPD. The cobalt oxyhydroxide with attached nickel hydroxides could be carried out by the cathodic EPD in the presence of nickel nitrate additive in IPA–water solution. The cathodic deposition of nickel hydroxide results from the chemical precipitation reaction between nickel ions and hydroxide ions. The generation of hydroxide ions is accompanied with the cathodic EPD resulting from the electrochemical reduction of nitrate ions and water molecules through the following reactions:42| NO3− + H2O + 2e− → NO2− + 2OH− | (1) |
| 2H2O + 2e− → H2 + 2OH− | (2) |
| Ni2+ + 2OH− → Ni(OH)2 ↓ | (3) |
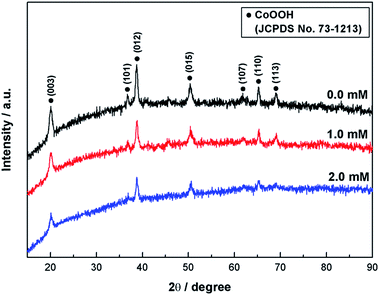 | ||
| Fig. 1 XRD patterns of cobalt oxyhydroxide samples with and without attached nickel hydroxides prepared by cathodic EPD in various concentrations of nickel nitrate. | ||
As a result, the nickel hydroxides could be coated on the cobalt oxyhydroxide particles being deposited during cathodic EPD. As revealed in Fig. 1, after deposition of nickel hydroxides on the surface of cobalt oxyhydroxide at higher concentrations of nickel nitrate additive, only the characteristic diffraction peaks of CoOOH could be detected and intensity of peaks becomes weak. This reflects that the deposited nickel hydroxide has ultra-low crystallinity and the CoOOH possesses almost unchanged crystal structural feature. Previous reports have indicated that the α-Ni(OH)2 is poorly crystalline with turbostratic structure. NO3− anions and solvents like water molecules may intercalate into the interlayer space of the Ni(OH)2 layers, leading to the formation of α-Ni(OH)2.49,50
Fig. 2 shows the TEM images of CoOOH sheets with and without attached Ni(OH)2 nanoparticles. The bare CoOOH sheet shown in Fig. 2a has well-defined hexagonal sheet morphology with an average size of approximately 450 nm. High-magnification TEM image shown in the inset of Fig. 2a reveals the porous structure of hexagonal sheet with pore size of about 4–8 nm. Fig. 2b shows the TEM image of CoOOH sheet with attached Ni(OH)2 nanoparticles. Clearly, the small Ni(OH)2 nanoparticles are coated on the surface and around the edge of hexagonal CoOOH sheet after cathodic EPD in the presence of 1 mM nickel nitrate. CoOOH is believed to have an excellent electrical conductivity, which has been exploited in the surface modification of Ni(OH)2 powder for enhanced electrode materials in alkaline electrolyte.15,16 Amorphous Ni(OH)2 may have large surface area for charge storage. However, the large surface area may come with more grain boundaries, leading to an increase in the electron transport resistance. The hexagonal CoOOH sheet with porous structure could provide more conductive paths for electron to travel through, facilitating the electrochemical charge-transfer reaction on the Ni(OH)2 nanoparticles. A close look at the TEM image of CoOOH sheet with attached Ni(OH)2 nanoparticles shown in the inset of Fig. 2b reveals that the porous structure of CoOOH sheet remains unchanged after cathodic EPD. Due to the similarity between Ni and Co, the displacement between Ni and Co may take place, leading to the formation of mixed oxide/hydroxide interface.51 Porous hexagonal sheet has been demonstrated to have positive effect on the capacitive behavior of electrode.52 In addition, the porous configuration may allow electrolyte to penetrate into the porous sheets freely, reducing the diffusion resistance of OH− within the bulk of sheets.
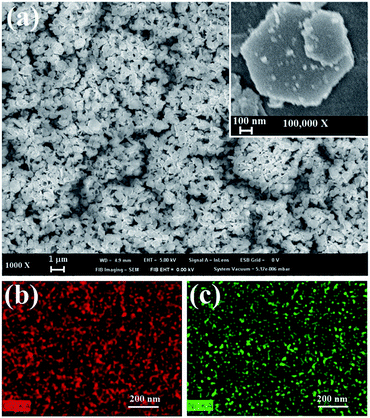
Fig. 3a shows the SEM image of CoOOH electrode with attached Ni(OH)2 nanoparticles prepared by cathodic EPD in the presence of 1 mM nickel nitrate. The zeta potential of CoOOH suspension in IPA–water solution containing 1 mM nickel nitrate additive was measured to be approximately 43 mV. Such high zeta potential is therefore beneficial to the dispersion of CoOOH sheets and consequently facilitates cathodic EPD under the applied electric field. As a result, the CoOOH sheets and nickel ions continue to deposit on the electrode, forming CoOOH sheets with attached Ni(OH)2 nanoparticles. The hexagonal sheets are randomly arranged on the electrode substrate, leaving space between sheets to accommodate the electrolyte. Fig. 3b and c show the elemental maps of cobalt and nickel determined by EDS, respectively. To avoid the interference of nickel from SS substrate, the EPD was carried out on a titanium substrate instead of SS substrate for EDS analysis. The nickel element is obtained from the composite film, reflecting the deposition of Ni(OH)2 on the surface of CoOOH sheets. A homogeneous distribution of nickel element indicates that the Ni(OH)2 nanoparticles could be self-assembled on the surface and around the edge of CoOOH sheet by a simple cathodic EPD method. Fig. 4 shows the variation in composition of CoOOH with attached Ni(OH)2 prepared by cathodic EPD in the presence of various nickel nitrate additives. The amount of Ni(OH)2 increases with increasing the concentration of nickel nitrate in the cathodic EPD. When deposited in 1 mM nickel nitrate, the content of Ni(OH)2 in the composite film is approximately 20 wt% calculated from the chemical formula and it reaches 45 wt% in the composite film deposited in 2 mM nickel nitrate. We can manipulate the content of Ni(OH)2 in the composite by adjusting the concentration of nickel nitrate at the same EPD time.
 | ||
| Fig. 4 Variation in the composition of CoOOH with attached Ni(OH)2 prepared by cathodic EPD in the presence of various nickel nitrate additives. | ||
Fig. 5 shows the cyclic voltammograms (CVs) of bare Ni(OH)2, bare CoOOH, and CoOOH with attached Ni(OH)2 electrodes. The composite electrodes were prepared by EPD in various concentrations of nickel nitrate. The bare Ni(OH)2 electrode was prepared similar to the composite electrode except that the EPD bath contained 1 mM nickel nitrate in the absence of CoOOH sheets. In addition to the oxygen evolution reaction (OER) at a potential more positive than 0.45 V, the bare Ni(OH)2 electrodes exhibit a pair of redox peaks resulting from the Faradaic redox reaction between Ni(OH)2 (Ni2+) and NiOOH (Ni3+). The redox reaction of bare Ni(OH)2 electrode in alkaline electrolyte can be described as follows:30
| Ni(OH)2 + OH− ↔ NiOOH + H2O + e− | (4) |
 | ||
| Fig. 5 Cyclic voltammograms of the bare Ni(OH)2, bare CoOOH, and CoOOH with attached Ni(OH)2 electrodes at a scan rate of 1 mV s−1 in 1 M KOH solution. | ||
The bare CoOOH electrode has an anodic peak at 0.45 V due to the OER and a weak cathodic peak at 0.12 V. Anodic peak of CoOOH electrode merges with OER and could not be clearly determined. The redox current may come from the redox reaction between Co(OH)2 (Co2+) and CoOOH (Co3+) which can be expressed as follows:30
| Co(OH)2 + OH− ↔ CoOOH + H2O + e− | (5) |
The CoOOH electrodes with attached nickel hydroxide nanoparticles show a couple of redox peaks similar to the bare Ni(OH)2 electrode, but their peak potentials shift to less positive potential. The incorporation of Co into the Ni(OH)2 lattices may cause the shift in peak potential.30 This means that a mixed hydroxide layer may be formed between the CoOOH and Ni(OH)2. Obviously, the CoOOH electrode with attached Ni(OH)2 nanoparticles deposited in 1 mM nickel nitrate has the highest current density in the cyclic voltammetry test, indicating the feasibility of rationally tuning the ratio of Co to Ni for optimizing the electrochemical performance of the composite electrodes. The higher the current density, the higher is the specific capacitance of the electrode. The bare CoOOH electrode has very low current density, suggesting that the current density of the composite electrodes comes mainly from the redox reaction of Ni(OH)2 and mixed hydroxide. The specific capacitance of CoOOH could be enhanced by attaching with Ni(OH)2 nanoparticles. This reflects that the porous hexagonal CoOOH sheets may facilitate the transport of electron and electrolyte through the porous channels in the CoOOH sheets. Ni(OH)2 with nano-sized particles can shorten the diffusion paths in the solid phase, leading to an increase in the effective surface area for charge storage through the redox reactions.
Fig. 6 shows the galvanostatic discharging curves of bare Ni(OH)2, bare CoOOH, and CoOOH with attached Ni(OH)2 electrodes at a current density of 1 A g−1. A linear potential profile of the bare CoOOH electrode indicates the pseudocapacitance behavior, resulting from the weak reduction peak in the CV curve. The CoOOH/Ni(OH)2 and bare Ni(OH)2 electrodes exhibit a potential plateau, significantly differing from the bare CoOOH electrode. The potential plateau arises from the Faradaic reaction between Ni(OH)2/Ni1−xCox(OH)2 and NiOOH/Ni1−xCoxOOH (where x is between 0 and 1). The elapsed time of the composite electrodes from the discharge beginning is considerably extended compared to that of bare CoOOH electrode at a discharge current density of 1 A g−1. This result indicates that the composite electrodes could provide much more active sites for Faradaic reaction to occur, leading to an increase in the specific capacitance. The specific capacitance of electrodes is determined by measuring the elapsed time while discharging the electrodes at a constant current density to the cut-off potential. The specific capacitance of electrodes during galvanostatic test can be calculated by the following equation:
| Csp = i × Δt/ΔV | (6) |
 | ||
| Fig. 6 Galvanostatic discharging curves of the bare Ni(OH)2, bare CoOOH, and CoOOH with attached Ni(OH)2 electrodes at a current density of 1 A g−1. | ||
High-performance electrochemical supercapacitors are expected to bridge the gap between EDLCs and traditional batteries to form fast charging/discharging energy-storage devices. Thus, it is essential for supercapacitors to exhibit better performance at high-rate charging and discharging circumstances. Fig. 7 shows the variation in specific capacitance of electrodes associated with the discharge current density. There is a little influence of current density on the specific capacitance of bare CoOOH electrode due to its high electrical conductivity. The influence of current density on specific capacitance of composite electrodes is enlarged by increasing the loading amount of Ni(OH)2 nanoparticles on CoOOH. Large amounts of Ni(OH)2 tend to form large aggregates on the CoOOH sheets, unfavorable for transport of electron and electrolyte. The bare Ni(OH)2 electrode shows the lowest rate capability, largely due to its aggregation and poor electrical conductivity. Coating CoOOH or Ni on the Ni(OH)2 surface is required to compensate for the lack of electrical conductivity, leading to a significantly improvement in the electrochemical performance of bare Ni(OH)2 materials.15,16 The specific capacitance of CoOOH with attached nickel hydroxide electrode (deposited in 1 mM nickel nitrate) could reach 1537 F g−1 at a low discharge current density of 1 A g−1, which still remains as high as 1140 F g−1 at a high discharge current density of 10 A g−1. It is noted that a large specific surface does not always ensure high specific capacitance because not all the surface area is available for electrolyte access. Randomly arranged hexagonal CoOOH sheets are developed using cathodic EPD to fulfill the requirements of high capacitance and rate performance. In addition to the amount of electrolyte-accessible surface area, electroactive sites available for charge storage and redox reaction should be considered.
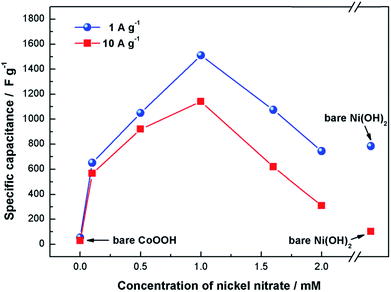 | ||
| Fig. 7 Variation in specific capacitance of the bare Ni(OH)2, bare CoOOH, and CoOOH with attached Ni(OH)2 electrodes associated with the discharge current density. | ||
As illustrated in Fig. 8, the conductive CoOOH scaffold composed of porous sheet networks is essential for electrically connecting the electroactive sites in Ni(OH)2 nanoparticles to improve the utilization of the active material and mitigate the charge-transfer resistance of Faradaic reaction. In addition, the porous hexagonal sheets allow the penetration of electrolyte through the porous channels in the sheets and thus shorten the diffusion paths in both the solid and liquid phases.
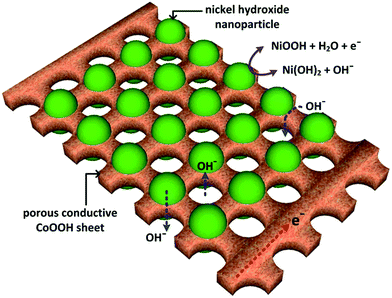 | ||
| Fig. 8 Schematic illustration for the enhanced capacitive behavior of porous hexagonal CoOOH sheet with attached Ni(OH)2 nanoparticles. | ||
Fig. 9 shows the Nyquist plots of bare Ni(OH)2, bare CoOOH, and CoOOH with attached Ni(OH)2 electrodes at an open-circuit potential of approximately 0.33 V. The CoOOH with attached Ni(OH)2 nanoparticles was prepared in the presence of 1 mM nickel nitrate. In a high-frequency range, the intercept at the Z′-axis is an Ohmic resistance primarily resulting from the electrolyte, active material, current collector, and active material/current collector interface. Ohmic resistance values for the Ni(OH)2, CoOOH, and Ni(OH)2/CoOOH electrodes were measured to be approximately 1.82, 1.10, and 1.31 Ω, respectively. The good electrical conductivity of bare CoOOH electrode is evident from the lower Ohmic resistance. The semicircle in the mid-frequency range can be attributed to the charge-transfer resistance and the electric double-layer capacitance on the surface of active materials. The charge-transfer resistance of bare CoOOH electrode is quite small, an indication of good electrical conductivity. The good conductive framework allows for fast electron conduction from the current collector to the active sites to facilitate the Faradaic reactions. Thus, the charge-transfer resistance of CoOOH with attached Ni(OH)2 electrode could be considerably reduced as compared with bare Ni(OH)2 electrode. In addition, a straight line inclined at a finite angle from the vertical is observed for each electrode in the low-frequency range, primarily due to electric double-layer capacitor of the porous electrodes.
Fig. 10 shows the cycle-life stability of bare Ni(OH)2, bare CoOOH, and CoOOH with attached Ni(OH)2 electrodes. The bare CoOOH electrode shows a very stable cycle life over 3000 cycles, reflecting the high stability of CoOOH in 1 M KOH electrolyte. The bare Ni(OH)2 electrode exhibits a poor stability during repeated charge and discharge cycles. As is well known, α-Ni(OH)2 is a metastable compound with turbostratic structure leading to a poor electrochemical stability in KOH electrolyte. Interestingly, the CoOOH with attached Ni(OH)2 nanoparticles (deposited in 1 mM nickel nitrate) shows a stable cycle-life performance compared with bare Ni(OH)2, about 82% of its initial capacitance remains after 3000 galvanostatic charge and discharge cycles at 10 A g−1. The enhanced cycle-life stability of the composite electrode may be due to the diffusion of Co into host Ni(OH)2 that suppresses the damages of lattices during cycling. In addition, the porous hexagonal CoOOH sheets are randomly deposited on the electrode substrate, leaving space between sheets to accommodate the electrolyte and mitigate the damage of electrode by repeated expansion and contraction of active materials during charge/discharge tests.
 | ||
| Fig. 10 Cycle-life stability of bare Ni(OH)2, bare CoOOH, and CoOOH with attached Ni(OH)2 electrodes. | ||
Conclusions
The porous hexagonal CoOOH sheets could be prepared by a simple chemical precipitation method in KOH solution as a conductive scaffold for supporting the Ni(OH)2 nanoparticles. The CoOOH sheets remained porous after attaching the Ni(OH)2 nanoparticles on their surface by the cathodic EPD methods. The amount of Ni(OH)2 nanoparticles on the CoOOH surface could be manipulated by tuning the concentration of nickel nitrate additive in the EPD bath. CV results showed that the redox peaks of CoOOH with attached Ni(OH)2 nanoparticles were shifted to less positive potential compared with those of bare Ni(OH)2 electrode, reflecting the formation of mixed Co–Ni hydroxide layers. The CoOOH with attached Ni(OH)2 nanoparticles deposited in 1 mM nickel nitrate showed the highest specific capacitance and superior cycle-life stability. The enhanced capacitive behavior might be due to the porous hexagonal CoOOH sheets that act as a current collector for facilitating the transport of electron and accommodate the large volume of liquid electrolyte to easy transport of ions. As a result, the proposed strategy can be exploited to construct the CoOOH/Ni(OH)2 composite electrodes for promising application in high-performance supercapacitors.Acknowledgements
The authors acknowledge financial support from the Ministry of Science and Technology, Taiwan (Grant no.: MOST 103-2221-E-151-057-MY3 and 103-3113-E-007-005).Notes and references
- B. E. Conway, J. Electrochem. Soc., 1991, 138, 1539–1548 CrossRef CAS PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- E. Frackowiak and F. Béguin, Carbon, 2001, 39, 937–950 CrossRef CAS.
- E. Frackowiak, Phys. Chem. Chem. Phys., 2007, 9, 1774–1785 RSC.
- Q. Lu, J. G. Chen and J. Q. Xiao, Angew. Chem., Int. Ed., 2013, 52, 1882–1889 CrossRef CAS PubMed.
- C. Yuan, H. B. Wu, Y. Xie and X. W. Lou, Angew. Chem., Int. Ed., 2014, 53, 1488–1504 CrossRef CAS PubMed.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- S. Liu, S. Sun and X.-Z. You, Nanoscale, 2014, 6, 2037–2045 RSC.
- X. Zhao, B. M. Sanchez, P. J. Dobson and P. S. Grant, Nanoscale, 2011, 3, 839–855 RSC.
- X. Rui, H. Tan and Q. Yan, Nanoscale, 2014, 6, 9889–9924 RSC.
- F. Tao, Y.-Q. Zhao, G.-Q. Zhang and H.-L. Li, Electrochem. Commun., 2007, 9, 1282–1287 CrossRef CAS PubMed.
- K. C. Liu and M. A. Anderson, J. Electrochem. Soc., 1996, 143, 124–130 CrossRef CAS PubMed.
- C. Lin, J. A. Ritter and B. N. Popov, J. Electrochem. Soc., 1998, 145, 4097–4103 CrossRef CAS PubMed.
- L.-B. Kong, M. Liu, J.-W. Lang, Y.-C. Luo and L. Kang, J. Electrochem. Soc., 2009, 156, A1000–A1004 CrossRef CAS PubMed.
- R. D. Armstrong, G. W. D. Briggs and E. A. Charles, J. Appl. Electrochem., 1988, 18, 215–219 CrossRef CAS.
- H. H. Law and J. Sapjeta, J. Electrochem. Soc., 1989, 136, 1603–1606 CrossRef CAS PubMed.
- M. Oshitani, H. Yufu, K. Takashima, S. Tsuji and Y. Matsumaru, J. Electrochem. Soc., 1989, 136, 1590–1593 CrossRef CAS PubMed.
- Z. Jiang, Z. Li, Z. Qin, H. Sun, X. Jiao and D. Chen, Nanoscale, 2013, 5, 11770–11775 RSC.
- J. Han, K. C. Roh, M. R. Jo and Y.-M. Kang, Chem. Commun., 2013, 49, 7067–7069 RSC.
- L. Huang, D. Chen, Y. Ding, S. Feng, Z. L. Wang and M. Liu, Nano Lett., 2013, 13, 3135–3139 CrossRef CAS PubMed.
- X. Liu, J. Huang, X. Wei, C. Yuan, T. Liu, D. Cao, J. Yin and G. Wang, J. Power Sources, 2013, 240, 338–343 CrossRef CAS PubMed.
- X. Sun, G. Wang, H. Sun, F. Lu, M. Yu and J. Lian, J. Power Sources, 2013, 238, 150–156 CrossRef CAS PubMed.
- Z. Gao, J. Wang, Z. Li, W. Yang, B. Wang, M. Hou, Y. He, Q. Liu, T. Mann, P. Yang, M. Zhang and L. Liu, Chem. Mater., 2011, 23, 3509–3516 CrossRef CAS.
- J. Huang, T. Lei, X. Wei, X. Liu, T. Liu, D. Cao, J. Yin and G. Wang, J. Power Sources, 2013, 232, 370–375 CrossRef CAS PubMed.
- N. Yulian, L. Ruiyi, L. Zaijun, F. Yinjun and L. Junkang, Electrochim. Acta, 2013, 94, 360–366 CrossRef PubMed.
- L. Zhang, J. Wang, J. Zhu, X. Zhang, K. San Hui and K. N. Hui, J. Mater. Chem. A, 2013, 1, 9046–9053 CAS.
- B. Wang, Q. Liu, Z. Qian, X. Zhang, J. Wang, Z. Li, H. Yan, Z. Gao, F. Zhao and L. Liu, J. Power Sources, 2014, 246, 747–753 CrossRef CAS PubMed.
- J. Zhao, J. Chen, S. Xu, M. Shao, D. Yan, M. Wei, D. G. Evans and X. Duan, J. Mater. Chem. A, 2013, 1, 8836–8843 CAS.
- P. Xu, K. Ye, D. Cao, J. Huang, T. Liu, K. Cheng, J. Yin and G. Wang, J. Power Sources, 2014, 268, 204–211 CrossRef CAS PubMed.
- V. Gupta, S. Gupta and N. Miura, J. Power Sources, 2008, 175, 680–685 CrossRef CAS PubMed.
- G. Chen, S. S. Liaw, B. Li, Y. Xu, M. Dunwell, S. Deng, H. Fan and H. Luo, J. Power Sources, 2014, 251, 338–343 CrossRef CAS PubMed.
- S. Cheng, L. Yang, Y. Liu, W. Lin, L. Huang, D. Chen, C. P. Wong and M. Liu, J. Mater. Chem. A, 2013, 1, 7709–7716 CAS.
- K.-W. Nam, E.-S. Lee, J.-H. Kim, Y.-H. Lee and K.-B. Kim, J. Electrochem. Soc., 2005, 152, A2123–A2129 CrossRef PubMed.
- C. Guan, J. Liu, C. Cheng, H. Li, X. Li, W. Zhou, H. Zhang and H. J. Fan, Energy Environ. Sci., 2011, 4, 4496–4499 CAS.
- X. Wang, J. Liu, Y. Wang, C. Zhao and W. Zheng, Mater. Res. Bull., 2014, 52, 89–95 CrossRef CAS PubMed.
- W. Ni, B. Wang, J. Cheng, X. Li, Q. Guan, G. Gu and L. Huang, Nanoscale, 2014, 6, 2618–2623 RSC.
- J. Zhang, X. Wang, J. Ma, S. Liu and X. Yi, Electrochim. Acta, 2013, 104, 110–116 CrossRef CAS PubMed.
- J. Pu, Y. Tong, S. Wang, E. Sheng and Z. Wang, J. Power Sources, 2014, 250, 250–256 CrossRef CAS PubMed.
- M.-S. Wu, Y.-R. Zheng and G.-W. Lin, Chem. Commun., 2014, 50, 8246–8248 RSC.
- E. Hosono, S. Fujihara, I. Honma, M. Ichihara and H. Zhou, J. Power Sources, 2006, 158, 779–783 CrossRef CAS PubMed.
- Z. Gao, W. Yang, Y. Yan, J. Wang, J. Ma, X. Zhang, B. Xing and L. Liu, Eur. J. Inorg. Chem., 2013, 2013, 4832–4838 CrossRef CAS.
- X. Lu, X. Huang, S. Xie, T. Zhai, C. Wang, P. Zhang, M. Yu, W. Li, C. Liang and Y. Tong, J. Mater. Chem., 2012, 22, 13357–13364 RSC.
- H. Chen, L. Hu, M. Chen, Y. Yan and L. Wu, Adv. Funct. Mater., 2014, 24, 934–942 CrossRef CAS.
- J.-W. Lang, L.-B. Kong, W.-J. Wu, Y.-C. Luo and L. Kang, Chem. Commun., 2008, 4213–4215 RSC.
- H. Jiang, T. Zhao, C. Li and J. Ma, J. Mater. Chem., 2011, 21, 3818–3823 RSC.
- J. Liu, C. Cheng, W. Zhou, H. Li and H. J. Fan, Chem. Commun., 2011, 47, 3436–3438 RSC.
- T. Alammar, O. Shekhah, J. Wohlgemuth and A.-V. Mudring, J. Mater. Chem., 2012, 22, 18252–18260 RSC.
- V. Pralong, A. Delahaye-Vidal, B. Beaudoin, B. Gerand and J. M. Tarascon, J. Mater. Chem., 1999, 9, 955–960 RSC.
- L. Dong, Y. Chu and W. Sun, Chem.–Eur. J., 2008, 14, 5064–5072 CrossRef CAS PubMed.
- L. Xu, Y.-S. Ding, C.-H. Chen, L. Zhao, C. Rimkus, R. Joesten and S. L. Suib, Chem. Mater., 2007, 20, 308–316 CrossRef.
- F. Allegretti, G. Parteder, M. G. Ramsey, S. Surnev and F. P. Netzer, Surf. Sci., 2007, 601, L73–L76 CrossRef CAS PubMed.
- J. Pu, J. Wang, X. Jin, F. Cui, E. Sheng and Z. Wang, Electrochim. Acta, 2013, 106, 226–234 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |