Large disk electrodes of Ti/TiO2-nanotubes/PbO2 for environmental applications†
Dayanne Chianca de Mouraa,
Mónica Cerro-Lópezb,
Marco Antonio Quirozb,
Djalma Ribeiro da Silvaa and
Carlos Alberto Martínez-Huitle*a
aFederal University of Rio Grande do Norte, Institute of Chemistry, Lagoa Nova – CEP 59.072-970, RN, Brazil. E-mail: carlosmh@quimica.ufrn.br
bUniversidad de las Américas Puebla. Grupo de Investigación en Energía y Ambiente. ExHda. Sta. Catarina Martir s/n, Cholula 72820, Puebla, Mexico
First published on 16th March 2015
Abstract
Large disk electrodes of Ti/TiO2-nanotubes/PbO2 (65 cm2 of geometrical area) were successfully synthesized by anodization and electrodeposition procedures. Characterization of anodes was performed by SEM, EDS, AFM and electrochemical measurements, aiming towards environmental applications. PbO2, an electrocatalytic material, promotes the production of strong oxidising species (hydroxyl radicals) that can be used for decontamination. Electrochemical treatment of synthetic dye effluent (2 L) containing 250 mg L−1 of Acid Blue 113 dye (AB 113) was performed using a disk Ti/TiO2-nanotubes/PbO2 anode and an electrochemical flow cell. More than 85% of organic matter was removed by applying current densities of 20, 40 and 60 mA cm−2. Moreover, colour decay achieved values of 60%, 90% and 100%, depending on the applied current density. Alternatively, this study allows us to understand how nanomaterials have prevented the corrosion phenomena on the anode surface (Pb2+ pollution) due to the homogeneous migration of PbO2 within the TiO2 nanotubes previously formed on the Ti support.
Introduction
Lead dioxide coatings on inert substrates such as titanium and carbon now offer new opportunities as an anode material for environmental applications. It is now recognised that electrodeposition allows the preparation of stable coatings with different phase structures and a wide range of surface morphologies.1 In addition, substantial modifications in the physical properties and catalytic activities of the coatings are possible through doping and fabrication of nanostructured deposits or composites.1,2 Lead dioxide can be electrodeposited onto inert substrates such as titanium or carbon from a number of media in which Pb(II) is soluble.2,3 These electrodes can only be used in applications that require a rather positive potential for electrochemical oxidation (EO). If a positive potential is applied, the coating will be protected from corrosion; but, at any significantly negative potential, cathodic reduction and dissolution of the lead dioxide coating is expected.4,5 Ti is the usual substrate for PbO2 coatings even when adhesion is a problem yet.6–8 Ti is usually in the form of a plate or expanded metal mesh, which must be pre-treated before the anodic plating process in order to remove any existing TiO2 scales from the surface to roughen the surface and to prevent passivation. This pre-treatment commonly involves sandblasting, alkaline degreasing followed by etching in heated oxalic acid or HCl for at least 30 min. However, pre-treatment is often insufficient, and hence various thin undercoats on Ti before deposition of the PbO2 have been proposed—gold, platinum, tin dioxide, TiO2/Ta2O5, PtOx and TiO2/RuO2.8–12 The main problem is that Ti self-generates an oxide layer that affects many of its properties such as charge transport and ability to adhere deposits on its surface. However, some research groups have recently developed nanostructured materials13,14 of TiO2 such as nanotubes, nanorods and nanowires, which act as a support to deposit PbO2.15–17 The unique properties of high aspect ratio TiO2 nanotubes include large surface area, high cation exchangeability, high catalytic activity, easy separation and recyclability.18 Three popular synthesizing techniques for TiO2 nanotubes have been investigated in recent years, including template-assisted, electrochemical anodization and hydrothermal treatment.19–24 The method of electrochemical anodization is based on the anodization of titanium foil to obtain nanoporous TiO2.25 Essentially, TiO2 nanotubes grow on the Ti surface with ordered arrangement and high aspect ratio, the dimensions of nanotubes formed are controlled by varying the electrolyte composition, applied voltage, pH and anodizing time.26,27 TiO2 nanotubes electrodes have superior photo-reactivity, non-toxicity, long term stability, high corrosion resistance and are available at low cost.28–30 These features offer a useful method to fabricate nanoscale architectures of metal oxides using this method. In the case of PbO2, it clearly emerges as an attractive material to be used anode for the direct oxidation of organic compounds due to its high oxygen evolution potential, low price, relative stability under the high positive potentials required and stability at high temperatures.31 The formation and growth of PbO2 inside TiO2 nanotubes have been already developed and used for environmental applications, but no significant dimensions of geometrical area have been produced.32 Therefore, the preparation of a more stable PbO2 anode on a good support such as TiO2 nanotubes could increase the economic value and accelerate the practical applications of these electrocatalytic materials. Herein, we report the first general preparation strategy for the synthesis of TiO2 nanotube arrays to deposit PbO2 in order to obtain large-disk electrodes. These synthesized large PbO2 anodes show predominant electrochemical performances and are promising tools for the next generation of electrocatalytic materials for environmental applications.Experimental
Preparation of Ti/TiO2-nanotubes and Ti/TiO2-nanotubes/PbO2
All the electrochemical deposition experiments were performed with a MINIPA MPL-3305 (São Paulo, Brazil) power supply. TiO2 nanotubes electrode was prepared according to the anodic oxidation method reported by Cerro-López and co-workers.32 The electrochemical procedure used a polished Ti disk with 10 cm diameter (1.8 mm thick and nominal surface area of 63.5 cm2). Before anodization of the Ti substrate (0.1 mm thick), the sample was successively sonicated in acetone, ethanol, and methanol for 15 minutes. Then, the anodizing process was performed using a two-electrode system (Ti disk serving as the anode and steel serving as the cathode) in a mixture of glycerol and ultrapure water in a 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (v/v) containing 0.5 wt% NaF and 0.2 M Na2SO4 as the support electrolyte by applying 30 V for 2 h (Fig. S1 of ESI†). After this procedure, the Ti/TiO2-nanotubes disk was immediately picked up from the electrolyte, rinsed with ultrapure water and finally dried. The electrodeposition of PbO2 onto a Ti/TiO2-nanotubes disk array was achieved using a galvanostatic method using a solution of 0.25 M Pb(NO3)2 + 0.1 M HNO3 by applying 30 mA cm−2 for 10 min at 25 °C (see Fig. S2 (ESI†)). No gas bubbling was involved.
1 ratio (v/v) containing 0.5 wt% NaF and 0.2 M Na2SO4 as the support electrolyte by applying 30 V for 2 h (Fig. S1 of ESI†). After this procedure, the Ti/TiO2-nanotubes disk was immediately picked up from the electrolyte, rinsed with ultrapure water and finally dried. The electrodeposition of PbO2 onto a Ti/TiO2-nanotubes disk array was achieved using a galvanostatic method using a solution of 0.25 M Pb(NO3)2 + 0.1 M HNO3 by applying 30 mA cm−2 for 10 min at 25 °C (see Fig. S2 (ESI†)). No gas bubbling was involved.
Surface characterization
Surface morphology of Ti/TiO2-nanotubes and Ti/TiO2-nanotubes/PbO2 deposits was characterized using a scanning electron microscope (SEM, TESCAN VEGA model LSU at a 30 kV acceleration voltage), energy-dispersive X-ray spectroscopy (EDS, Shimadzu Electron Probe Microanalyzer EPMA-1720) and atomic force microscopy (AFM), while crystalline phases were determined using an X-ray diffractometer (DRX Bruker model D8Discover) using Cu Kα (λ = 1.54 Å) radiation. Electrochemical measurements were obtained using an AutoLab 302N (Metrohm workstation). For the study of electrochemical characteristics of Ti/TiO2-nanotubes/PbO2 and service life performances, cyclic voltammetry (CV) and polarization measurements of PbO2 deposits were performed in 1.0 mol L−1 H2SO4 between 0.5 and 3.5 V vs. Ag/AgCl (3 M) at 50 mV s−1.Electrochemical experiments
The electrocatalytic features of the Ti/TiO2-nanotubes/PbO2 electrode were tested during the EO of the dye solution by applying 20, 40 and 60 mA cm−2 of current density. EO experiments were performed using an electrolytic flow cell with a single compartment with parallel plate electrodes for treating 2 L of 250 mg L−1 of Acid Blue 113 dye (AB 113) at 25 °C (see Fig. S3†). The dye was obtained from a textile industry at Santiago de Chile (Chile) with no further purification prior to use. The characteristics of the dye Acid Blue 113 are shown in Table 1. Colour and chemical oxygen demand (COD) analyses were performed to evaluate the depollution performances. Moreover, analyte samples were subjected to GC-MS analysis using GC-FOCUS and MS-ISQ Thermo Scientific to identify the intermediates.Results and discussion
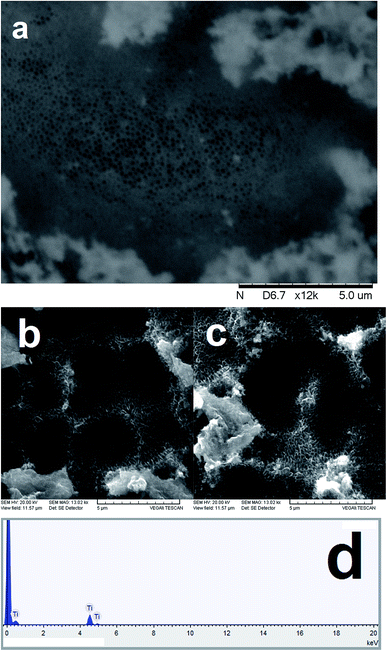
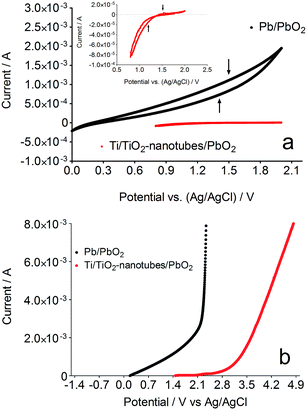
Ti/TiO2-nanotubes were successfully synthesized in a solution of glycerol and ultrapure water containing NaF and Na2SO4 by galvanostatic electrolysis with a potential of 30 V for 120 min. The surface morphology of deposits was observed by SEM, showing TiO2 nanotubes with diameters of approximately 100–200 nm as observed in the images. The TiO2 nanotubes grow vertically on the substrate and form a dense array, as shown in Fig. 1. Moreover, EDS measurements were obtained at different points along the TiO2 nanotubes, and the typical EDS spectrum is shown in Fig. 1b. The composition analysis shows that the Ti/O stoichiometry corresponds to TiO2, which demonstrates that the nanotubes were successfully synthesized. Furthermore, the AFM study reveals the profile of the lateral view of TiO2 nanotubes (see Fig. S4†), confirming the nanometric diameter of the tubes formed (see Fig. 1). In addition, PbO2 crystals were successfully deposited onto Ti/TiO2-nanotubes arrays, according to Fig. 2. Deposits were composed of orderly arranged tetragonal PbO2 crystals with sizes of about 15 μm (see inset and Fig. 2). These tetragonal crystals can assemble in tree form when the electrodeposition time is increased (see Fig. S5 (ESI†)). Fig. S6 (ESI†) clearly shows the growth of PbO2 crystals on the TiO2 nanotubes after 30 min of electrodeposition time. For this reason, the electrolysis time of 10 min is very important to obtain a homogenous PbO2 deposit. The side lengths of PbO2 are about 3.0–4.0 μm, and the thickness of the films was minimum, as shown in the inset in Fig. 2. EDS measurements were carried out at different points along the PbO2 film, and the typical EDS spectrum is shown in Fig. 2. The additional composition analysis showed that the crystals formed in the TiO2-nanotubes arrays consisted of pure PbO2. Fig. S7 (ESI†) shows the XRD pattern of the synthesized PbO2 crystals. All the peaks can be indexed to β-PbO2 phases. No peaks of any other phases or impurities were detected, which also revealed that pure PbO2 was obtained. The mechanism of formation of the PbO2 morphology during electrodeposition is discussed in ESI.† Herein, the performances of large TiO2-nanotubes/PbO2 anodes for EO were studied (see Fig. S8†). Nevertheless, the preliminary examination was performed by CVs of TiO2-nanotubes/PbO2 (shown in Fig. 2) recorded at 50 mV s−1 in 1.0 M H2SO4, as shown in Fig. 3. In Fig. 3, for each case, the 20th cycle sweeping from 0.0 V to 2.0 V is shown. In the negative scanning, a slight reduction signal is observed at about 1.4 V for the PbO2 deposit, and this can be attributed to the electroreduction of PbO2 to PbSO4 via the following electrochemical reaction: PbO2(s) + HSO4(aq) + 3H+ + 2e → PbSO4(s) + 2H2O. From the area under the cathodic signal, it can be clearly observed that the charge passed at the Ti/TiO2-nanotubes/PbO2 anode is several times less than that for the Pb/PbO2 anode even when both the anodes were treated by similar Pb deposition procedures.On the reverse scan, only relatively small anodic signals are observed for both the samples, as shown in Fig. 3, suggesting the well-known passivating effect of PbSO4. After several CV curves, the cathodic signal decreased in the successive cycles due to the reduced availability of PbO2 for electroreduction. After 40 cycles, the charge passed at the Ti/TiO2-nanotubes/PbO2 anode is about 50 times less than that passed at the Pb/PbO2 electrode, as shown in the inset of Fig. 3a. Therefore, the Ti/TiO2-nanotubes/PbO2 anode shows considerably better electrochemical properties than the Pb/PbO2 anode, and this may be attributed to the considerably larger surface area of the TiO2 nanostructures, providing considerably more open-edge morphologies and considerably larger network structures to the PbO2 crystals formed. Fig. 3b also shows linear polarization curves of the Pb/PbO2 and Ti/TiO2-nanotubes/PbO2 electrodes obtained in 1.0 M H2SO4 with a scan rate of 50 mV s−1. The curves are very different and show that the oxygen evolution potential increases from 0.0 V to 2.8 V versus Ag/AgCl (3.0 M) for Pb/PbO2 and Ti/TiO2-nanotubes/PbO2, respectively. This indicates that Pb/PbO2 has a high oxygen evolution overpotential, and consequently is a poor electrocatalyst for the oxygen evolution reaction (o.e.r.); however, the potential of the o.e.r. was shifted to a more positive potential when Ti/TiO2-nanotubes/PbO2 was employed, and consequently noticeably disfavoring the production of oxygen.33,34 This behavior indicates that the latter anode could exhibit good electrocatalytic properties for EO of organic pollutants in solution.31,33,34
EO experiments were performed in order to evaluate the electrocatalytic features of disk Ti/TiO2-nanotubes/PbO2 anodes to remove the organic load and color of a dye synthetic effluent with an electrochemical flow cell by applying a current density of 20, 40 and 60 mA cm−2. Fig. 4 shows color decay during the galvanostatic electrolysis of dye solutions containing 250 mg dm−3 AB 113. The removal changes were reasonably rapid, indicating that during the first treatment stages there are mechanisms involving dye oxidation to other simpler organics.33 The oxidation of this complex molecule can result in the formation of many intermediates by the elimination of chromophore groups prior to the production of aliphatic carboxylic acids and carbon dioxide.34,35 This can be explained because at the Ti/TiO2-nanotubes/PbO2 electrodes, ˙OH radicals formed by water oxidation (H2O → ˙OH + H+ + e−) can be either electrochemically oxidized to dioxygen (˙OH → 1/2O2 + H+ + e−) or contribute to the complete oxidation of the organic compounds, in this case, dyes (dyes + ˙OH → CO2 + H2O).36,37 According to Comninellis,38 this anode can be classified as a non-active anode, thus favoring the electrochemical combustion of organic pollutants. However, color removal decreased when the applied current density was increased. This behavior is due to the promotion of the oxygen evolution reaction37,38 when an increase in the applied current density was attained. It can also be confirmed by polarization curves, which demonstrated the higher production of oxygen at higher current values (see Fig. 3b). Nevertheless, % of COD values (ESI†) clearly demonstrated that the oxidation power of this non-active electrode is independent of applied current density at different electrolysis times (see inset in Fig. 4). This outcome is in agreement with the data reported by other authors for the anodic oxidation of dyes.33,39–42 During the electrolysis of synthetic dye effluent, the energy consumption (ESI†) seems to be proportional to the applied current density. For example, it increases from 39.2 to 70.3 kW h m−3 of effluent treated when the current density increases from 20 to 60 mA cm−2. Conversely, a higher energy was required when Pb/PbO2 was used as the anode (57 to 100 kW h m−3). After the EO of synthetic dye using the Ti/TiO2-nanotubes/PbO2 anode, by-products were identified by GC/MS. 1-Naphthalenol, naphthalene, 1,6-dimethyl-4-(1-methylethyl) and dibutyl phthalate were identified as intermediates, confirming the chromophore group cleavage as a first step followed by the formation of aromatic intermediates, giving lower coloration to the solution.41,42 The electrochemical stability of the disk Ti/TiO2-nanotubes/PbO2 was examined by subjecting an electrode to fixed current density measurements for prolonged electrolysis times. Fig. S9 (ESI†) shows the variation of Eappl as a function of time. As revealed from these data, there is almost no increase in the values of Eappl up to 120 h. Therefore, the disk Ti/TiO2-nanotubes/PbO2 composed of orderly arranged tetragonal PbO2 crystals showed high electrochemical stability for long-term applications. In addition, it is important to indicate that no pollution of Pb2+ was detected after longer times of electrolysis with the Ti/TiO2-nanotubes/PbO2 anode, in contrast with the results achieved when the Pb/PbO2 electrode was used. The release of Pb2+ was monitored by electroanalytical measurements;43 however, other techniques, such as ICP/MS and ICP/OES,44–46 can be used to evaluate this parameter.
Conclusions
In summary, the large PbO2 electrodes composed of orderly arranged TiO2 nanotubes were synthesized via a simple, rapid, and efficient electrochemical approach. Compared with Pb/PbO2 electrodes, the prepared Ti/TiO2-nanotubes/PbO2 can promote the remarkable enhancement in the performances of electrocatalytic materials for the treatment of wastewaters.39,40 The higher electrochemical activities of Ti/TiO2-nanotubes/PbO2 than those of Pb/PbO2 may be attributed to the larger surface area, more open-edge morphologies and larger network structures in the crystal body. This study will open a new approach in the search for new metal oxide structures from the preparation of TiO2 nanotubes, giving more stability to non-active anodes for electrochemical devices with excellent performances.Notes and references
- X. Li, D. Pletcher and F. C. Walsh, Chem. Soc. Rev., 2011, 40, 3879 RSC.
- D. Pletcher and F. C. Walsh, Industrial Electrochemistry, Chapman and Hall, London, 2nd edn, 1990 Search PubMed.
- G. Planté, Compt. Rend., 1859, 49, 402 Search PubMed; G. Planté, Compt. Rend., 1860, 50, 640 Search PubMed.
- A. M. Couper, D. Pletcher and F. C. Walsh, Chem. Rev., 1990, 90, 837 CrossRef CAS.
- A. J. Bard, R. Parsons and J. Jordan, Standard Potentials in Aqueous Solutions, Marcel Dekker, Inc., New York, 1985 Search PubMed.
- A. T. Kuhn, The Electrochemistry of Lead, Academic Press Inc. Ltd., London, 1st edn, 1979 Search PubMed.
- D. W. Wabner, R. Huss, F. Hindelang, H. P. Fritz and D. Missol, Z. Naturforsch., 1976, 31B, 45–50 CAS.
- D. W. Wabner, H. P. Fritz and R. Huss, Chem. Ing. Tech., 1977, 49, 329 CrossRef CAS.
- C. Comninellis and E. Plattner, J. Appl. Electrochem., 1982, 12, 399 CrossRef CAS.
- D. Devilliers, M. T. D. Thi, E. Mahe and Q. Le Xuan, Electrochim. Acta, 2003, 48, 4301 CrossRef CAS PubMed.
- M. Ueda, A. Watanabe, T. Kameyama, Y. Matsumoto, M. Sekimoto and T. Shimamune, J. Appl. Electrochem., 1995, 25, 817 CrossRef CAS.
- F. Hine, M. Yasuda, T. Iida, Y. Ogata and K. Hara, Electrochim. Acta, 1984, 29, 1447 CrossRef CAS.
- G. Cao, Nanostructures & Nanomaterials: Synthesis, Properties & Applications, Imperial College Press, London, 2004 Search PubMed.
- G. A. Ozin and A. C. Arsenault, Nanochemistry: A Chemical Approach to Nanomaterials, Royal Society of Chemistry, Cambridge, 2005 Search PubMed.
- R. Inguanta, S. Piazza and C. Sunseri, J. Electrochem. Soc., 2008, 155, K205 CrossRef CAS PubMed.
- P. Perret, T. Brousse, D. Bélanger and D. Guay, J. Electrochem. Soc., 2009, 156, A645 CrossRef CAS PubMed.
- P. N. Bartlett, T. Dunford and M. A. Ghanem, J. Mater. Chem., 2002, 12, 3130 RSC.
- A. E. Ruby Mohamed and S. Rohani, Energy Environ. Sci., 2011, 4, 1065 Search PubMed.
- Y. L. Pang, S. Lim, H. C. Ong and W. T. Chong, Appl. Catal., A, 2014, 481, 127 CrossRef CAS PubMed.
- D. Kowalski, D. Kim and P. Schmuki, Nano Today, 2013, 8, 235 CrossRef CAS PubMed.
- N. K. Shrestha, P. Schmuki, R. G. Compton and J. D. Wadhawan, Electrochemistry at TiO2 nanotubes and other semiconductor nanostructures, Electrochemistry, RSC Publishing, Cambridge, 2014, vol. 12, pp. 87–131 Search PubMed.
- S. Bauer, S. Kleber and P. Schmuki, Electrochem. Commun., 2006, 8, 1321 CrossRef CAS PubMed.
- M. Assefpour-Dezfuly, C. Vlachos and E. H. Andrews, J. Mater. Sci., 1984, 19, 3626 CrossRef CAS.
- J. M. Macak, H. Tsuchiya and P. Schmuki, Angew. Chem., Int. Ed., 2005, 44, 2100 CrossRef CAS PubMed.
- R. P. Antony, T. Mathews, C. Ramesh, N. Murugesan, A. Dasgupta, S. Dhara, S. Dash and A. K. Tyagi, Int. J. Hydrogen Energy, 2012, 37, 8268 CrossRef CAS PubMed.
- D. Gong, C. A. Grimes, O. K. Varghese, W. Hu, R. S. Singh, Z. Chen and E. C. Dickey, J. Mater. Res., 2001, 16, 3331 CrossRef CAS.
- S. Sreekantan, Z. Lockman, R. Hazan, M. Tasbihi, L. K. Tong and A. R. Mohamed, J. Alloys Compd., 2009, 485, 478 CrossRef CAS PubMed.
- N. Pugazhenthiran, S. Murugesan and S. Anandan, J. Hazard. Mater., 2013, 263, 541 CrossRef CAS PubMed.
- M. Plodinec, A. Gajovic, G. Jaksa, K. Zagar and M. Ceh, J. Alloys Compd., 2014, 591, 147 CrossRef CAS PubMed.
- Y. Tang, Z. Jiang, Q. Tay, J. Deng, Y. Lai, D. Gong, Z. Dong and Z. Chen, RSC Adv., 2012, 2, 9406 RSC.
- C. A. Martínez-Huitle and M. Panizza, Application of PbO2 Anodes for wastewater Treatment, in Advances in Chemistry Research: Applied Electrochemistry, Nova Science Publishers, Inc, New York, 2010, pp. 269–300 Search PubMed.
- M. Cerro-López, Y. Meas-Vong, M. A. Méndez-Rojas, C. A. Martínez-Huitle and M. A. Quiroz, Appl. Catal., B, 2014, 144, 174 CrossRef PubMed.
- C. A. Martínez-Huitle and E. Brillas, Appl. Catal., B, 2009, 87, 105 CrossRef PubMed.
- C. A. Martínez-Huitle and S. Ferro, Chem. Soc. Rev., 2006, 35, 1324 RSC.
- M. Panizza and G. Cerisola, Chem. Rev., 2009, 109, 6541 CrossRef CAS PubMed.
- B. Marselli, J. García-Gómez, P.-A. Michaud, M. A. Rodrigo and C. Comninellis, J. Electrochem. Soc., 2003, 150, D79 CrossRef CAS PubMed.
- C. Comninellis, Electrochim. Acta, 1994, 39, 1857 CrossRef CAS.
- C. Comninellis, A. Kapalka, S. Malato, S. A. Parsons, I. Poulios and D. Mantzavinos, J. Chem. Technol. Biotechnol., 2008, 83, 769 CrossRef CAS.
- M. Panizza and G. Cerisola, Appl. Catal., B, 2007, 75, 95 CrossRef CAS PubMed.
- E. Brillas and C. A. Martínez-Huitle, Appl. Catal., B, 2015, 166–167, 603 CrossRef CAS PubMed.
- C. K. C. Araújo, G. R. Oliveira, N. S. Fernandes, C. L. P. S. Zanta, S. S. L. Castro, D. R. da Silva and C. A. Martínez-Huitle, Environ. Sci. Pollut. Res., 2014, 21, 9777 CrossRef PubMed.
- J. H. Bezerra Rocha, M. M. Soares Gomes, E. V. dos Santos, E. C. Martins de Moura, D. Ribeiro da Silva, M. A. Quiroz and C. A. Martínez-Huitle, Electrochim. Acta, 2014, 140, 419 CrossRef PubMed.
- M. M. S. G. Eiband, K. C. A. Trindade, K. Gama, J. V. Melo, C. A. Martínez-Huitle and S. Ferro, J. Electroanal. Chem., 2014, 717–718, 213 CrossRef CAS PubMed.
- H. Lin, J. Niu, J. Xu, H. Huang, D. Li, Z. Yue and C. Feng, Environ. Sci. Technol., 2013, 47, 13039 CrossRef CAS PubMed.
- J. Niu, H. Lin, J. Xu, H. Wu and Y. Li, Environ. Sci. Technol., 2012, 46, 10191 CAS.
- H. Lin, J. Niu, S. Ding and L. Zhang, Water Res., 2012, 46, 2281 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra16723f |
| This journal is © The Royal Society of Chemistry 2015 |