A facile one-pot synthesis of L-DOPA imprinted silica nanospheres for chiral separation and in vitro controlled release†
Chang-Long Kaoa,
Yan-Fu Chena,
Ping-Chih Huangb,
Ching-Yun Hsub and
Chun-Hsiung Kuei*a
aDepartment of Chemistry, National Cheng Kung University, Tainan City 701, Taiwan, Republic of China. E-mail: kuei@mail.ncku.edu.tw; Fax: +886-6-2089092; Tel: +886-6-2089092
bDepartment of Chemical and Materials Engineering, Cheng Shiu University, Kaohsiung Country 83347, Taiwan, Republic of China
First published on 27th January 2015
Abstract
A one-pot methodology was successfully developed to synthesize chiral imprinted silica nanospheres with hollow and mesoporous structures. These silica nanospheres demonstrated high capacity and chiral selectivity for L- or D-3,4-dihydroxyphenylalanine (L- or D-DOPA) and possessed potency for L-DOPA controlled release.
Molecular imprinting is a promising procedure for producing tailor-made biological hosts with molecular recognition properties and selectivity.1 In the molecular imprinting process, functional monomers are self-assembled with the desired template molecule through covalent or non-covalent bonding and then polymerized with a cross-linker. Binding sites compatible with the template are generated after the removal of the template from the polymer matrix known as molecularly imprinted polymer (MIP). MIPs are mechanically and chemically stable, compared to natural biological receptors and enzymes.2 Therefore, tailor-made biological hosts synthesized by molecular imprinting can be operated in a wide range of practical conditions.3
Polyakov was the first pioneer who demonstrated molecular imprinting in silica in 1931.4 However, there is a problem for molecular imprinting in bulk silica, bulk silica is more rigid for molecular imprinting and slow diffusion through bulk silica gives poor access to imprinted sites.5 To reduce the diffusion distance can be a solution to this problem. Several studies have obtained good results to reduced the diffusion length by surface imprinting.6 Surface imprinting of organic molecules eliminate the diffusion length, but introduces the risk of creating closed imprinted cavities, which scarify the selectivity of size and shape. Recently, Chang, et al. have shown highly mesoporous molecularly imprinted silica can also overcome the diffusion problem.7 In addition, periodic mesoporous silicas (PMSs) have been created using a sol–gel approach involving a micelle template in solution media, to generate a mesoporous material.8 These materials have a closed packed network channel that increases accessibility to imprinted sites and allows easier flow. Besides, molecularly imprinted silica is more accessible, biocompatible and mechanically stronger for biological, industrial, and preparative applications. For example, silica powder can be easily packed into columns as separation media, or suspended in a biofluid or solvents as a sensor for drug carriers.9
However, a facile synthesis of chiral imprinted silica nanospheres with hollow and mesoporous structures has rarely reported. Herein we chose L-DOPA as the chiral template because it is an important precursor of dopamine, norepinephrine, and epinephrine known as catecholamines. L-DOPA is also used to treat Parkinsons' disease and dopamine responsive dystonia by increasing dopamine concentration. In this work, we successfully developed a one-pot methodology to synthesize chiral silica nanosphere imprinted with L-DOPA. We also chose D-DOPA as the compared template and L-tyrosine as test molecule. The synthetic process, the reaction can be easily monitored by the fluorescence spectrometer and Fourier transform infrared (FTIR) spectrometer. The obtained materials have high capacity and chiral selectivity for L-DOPA and possessed potency for L-DOPA controlled release. The synthesis process and identification of functionalized the hollow and mesoporous silica nanospheres were investigated (see ESI†). Synthetic procedures are shown in Scheme 1.
 | ||
| Scheme 1 Schematic procedure for synthesizing the L-DOPA imprinted silica nanospheres with proposed interactions between L-DOPA and functional groups. | ||
Instead of post-grafting synthesis, hollow and mesoporous spheres were formed and functionalized in a one-pot and in situ sol–gel process by site-selectively molecular imprinting, which confined the location of L-DOPA to the cavity. The process of creating imprinted nanospheres (INSs) is briefly described blow, L-DOPA was used as the target template imprinted or recognized by 4-formylphenylboronic acid (pB) and (3-aminopropyl)triethoxysilane (APTES), cetyl trimethylammonium bromide (CTAB) and tetraethyl orthosilicate (TEOS) were used to form porous structures. Then L-DOPA was confined to the space by alkaline co-condensation. Finally, the INSs were dried and extracted by HCl, water and ethanol. The same procedure was used to synthesize non-imprinted nanospheres (NINSs) only without template. NINSs were used as control experiment.
James and Fossey et al. have showed that boronic acid has high affinity for diol compounds.10 Since L-DOPA contains diol moieties, it can be recognized by boronic acid. Therefore, the characteristic fluorescent emission spectrum of pB was measured every 10 minutes during the reaction (ESI Fig. 1†). The reaction also can be monitored by FTIR. After L-DOPA was recognized by pB and APTES, the aldehyde group became an imine group by condensation with APTES. This process can be easily monitored by FTIR (ESI Fig. 2†). The imine formation can also be confirmed by solid-state 13C nuclear magnetic resonance (NMR) (ESI Fig. 3†).
Stable, hollow, porous and monodispersed nanospheres were obtained after drying and extraction. The morphology of both INSs and NINSs was characterized by transmission electron microscopy (TEM). The TEM images (Fig. 1) showed that INSs and NINSs have average diameters of about 360 nm. The morphology of the two types of sphere might be result from the imprinting effect, the high nucleation rate and slow sequence growth of seeds.11 The size distributions of spheres were characterized by dynamic light scattering (DLS). INSs and NINSs both have almost monodispersed distributions in INSs and NINSs as shown in DLS (Fig. 1).
 | ||
| Fig. 1 TEM of morphologies and DLS of the size distributions of spheres (a) INSs and (b) NINSs. All scale bars are 100 nm in TEM images. | ||
The porous structures were characterized by small-angle X-ray diffraction (SAX, ESI Fig. 4†), INSs and NINSs both have one intense peak indexed to (100) diffraction, which is characteristic of SBA-15, a high ordered and hexagonally arranged pore structure. The characteristic peaks indexed to (110) and (200) diffractions are less resolved because organic silanes (APTES) would disturb the self-assembly of the surfactant micelles and the precursors.12
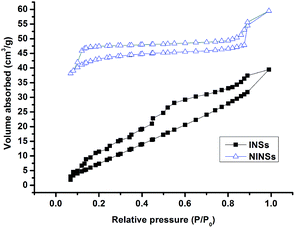
The porosity of INSs and NINSs was investigated by a nitrogen adsorption–desorption experiment (Fig. 2). The obtained type IV curves and indicated pore size in the mesoporous range. The Brunauer–Emmett–Teller (BET) surface area of INSs is 294.67 m2 g−1 and that of NINSs is 290.32 m2 g−1, as obtained from the nitrogen isotherm. The Barrat–Joyner–Halenda (BJH, Fig. 3) pore size distribution shows uniform mesopores with an average diameter of 1.90 nm for INSs and 2.34 nm for NINSs. BJH data might indicate that imprinting effect decreases pore size. The pore volumes were estimated to be 0.20 cm3 g−1 for INSs and 0.19 cm3 g−1 for NINSs.
 | ||
| Fig. 3 Distributions of pore size of INSs and NINSs characterized by BET adsorption–desorption isotherms. | ||
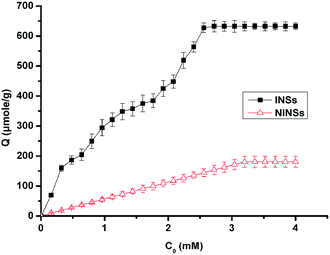
The imprinting effect is as usually evaluated by specific adsorption capacity that can indicate the rebinding specificity and imprint amount. Therefore, the molecular recognition properties and binding capacity of INSs were further investigated by static and kinetic rebinding tests. In order to investigate the binding characteristics of INSs and NINSs, the binding isotherm of L-DOPA to INSs and NINSs was studied in the 0 to 4 mM range of initial concentration of L-DOPA, which were shown in the isotherm binding vs. L-DOPA concentration (Fig. 4). The result shows that the rebinding capacities for L-DOPA on INSs and NINSs increase with the increase of L-DOPA concentration from 0 to 2.5 mM. However, when initial L-DOPA concentration was above 3 mM, the adsorption curve became flat and reached saturation. The result also shows that INSs have higher binding amounts then NINSs within the whole concentration range. As well as Fig. 4 indicates that INSs has maximum binding capacity about 632 μmole g−1 of L-DOPA and NINSs has relatively low binding capacity about 180 μmole g−1, which prove the molecularly imprinted effect.
Since INSs have better imprinted molecularly imprinted effect, INSs were chosen for further controlled release study. As we know that L-DOPA is also used to treat Parkinsons' disease and dopamine responsive, INSs were used as controlled release carriers for L-DOPA. The in vitro controlled released profiles as a function of pH of the media were shown in Fig. 5. At pH 7.4 and 6, the release of L-DOPA of INSs was 95% within 1.5 hours and more completely then that at pH 9. At pH 7.4 and 6, the interactions between L-DOPA and functional groups are proposed as hydrogen bondings unlike ionic interactions at pH 9. At pH 9 shows stronger binding then that at pH 7.4 and 6.13,14 Initial quick release of L-DOPA might be related to physical or non-specific interactions.
In order to further investigate the chiral and specific recognition of INSs, D-DOPA and L-tyrosine were used as structure analogue to investigate the specific recognition of INSs (Fig. 6). We also used D-DOPA as compared template to show the specific recognition of INSs. Fig. 6(a) shows L-DOPA used as the template in INSs and NINSs, noted as L-INSs and L-NINSs. Fig. 6(b) shows D-DOPA used as the template in INSs and NINSs, noted as D-INSs and D-NINSs. As results shown in Fig. 6(a) and (b), the L-INSs and D-INSs show the highest recognition for L-DOPA and D-DOPA respectively, which indicates INSs have chiral recognition ability and high affinity. Fig. 6 also shows L-INSs and D-INSs both have lower affinity to L-tyrosine.
 | ||
| Fig. 6 Binding selectivity of INSs and NINSs. (a) L-DOPA as the template. (b) D-DOPA as the template. | ||
In summary, a one-pot methodology was developed for synthesizing chiral imprinted silica nanospheres and the reaction can be easily monitored by fluorescence spectrometer and FTIR. The in vitro controlled release show pH dependent property that can extend the application of imprinted nanospheres. Furthermore, these chiral imprinted nanospheres shows superior high capacity and selectivity for L-DOPA and D-DOPA respectively. The further work is in progress to design more novel, magnetic, reliable, and high sensitive imprinted nanospheres. The properties and applications of the new materials are being studied.
Acknowledgements
This work was financial supported by National Science Council (NSC. 96-2113-M-006-016-MY2, NSC. 98-2113-M-006-007-MY2 and NSC. 100-2113-M-006-001-MY3). The authors are grateful to Mr Pin-Wei Juan from Professor Chen-Sheng Yeh's Lab for generous help for BET analysis in this work.Notes and references
- L. Chen, S. Xu and J. Li, Chem. Soc. Rev., 2011, 40, 2922–2942 RSC.
- Y. Hoshino, H. Koide, T. Urakami, H. Kanazawa, T. Kodama, N. Oku and K. J. Shea, J. Am. Chem. Soc., 2010, 132, 6644–6645 CrossRef CAS PubMed.
- (a) Y. Ben-Amram, M. Riskin and I. Willner, Analyst, 2010, 135, 2952–2959 RSC; (b) L. Peng, Y. Wang, H. Zeng and Y. Yuan, Analyst, 2011, 136, 756–763 RSC; (c) J. Liu, K. Yang, Q. Deng, Q. Li, L. Zhang, Z. Lianga and Y. Zhang, Chem. Commun., 2011, 47, 3969–3971 RSC; (d) X. Shen, L. Zhu, N. Wang, L. Yec and H. Tang, Chem. Commun., 2012, 48, 788–798 RSC.
- M. V. Polyakov, Zh. Fiz. Khim., 1931, 2, 799–804 Search PubMed.
- D. R. Kryscio and N. A. Peppas, Acta Biomater., 2012, 8, 461–473 CrossRef CAS PubMed.
- (a) M. Frasconi, R. Tel-Vered, M. Riskin and I. Willner, Anal. Chem., 2010, 82, 2512–2519 CrossRef CAS PubMed; (b) Q.-Q. Gai, F. Qu, Z.-J. Liu, R.-J. Dai and Y.-K. Zhang, J. Chromatogr. A, 2010, 31, 5035–5042 CrossRef PubMed; (c) J.-Q. Xue, D.-W. Li, L.-L. Qu and Y.-T. Long, Anal. Chim. Acta, 2013, 13, 57–62 CrossRef PubMed.
- B. M. Jung, M. S. Kim, W. J. Kim and J. Y. Chang, Chem. Commun., 2010, 46, 3699–3701 RSC.
- (a) W. Wang, J. E. Lofgreen and G. A. Ozin, Small, 2010, 6, 2634–2642 CrossRef CAS PubMed; (b) F. Tang, L. Li and D. Chen, Adv. Mater., 2012, 24, 1504–1534 CrossRef CAS PubMed.
- (a) B. T. S. Bui and K. Haupt, Anal. Bioanal. Chem., 2010, 398, 2481–2492 CrossRef CAS PubMed; (b) M. J. Whitcombe, I. Chianella, L. Larcombe, S. A. Piletsky, J. Noble, R. Porter and A. Horgan, Chem. Soc. Rev., 2011, 40, 1547–1571 RSC; (c) J. Yin, Y. Cui, G. Yang and H. Wang, Chem. Commun., 2010, 46, 7688–7690 RSC; (d) S. Azodi-Deilamia, M. Abdoussa and S. R. Seyedi, Cent. Eur. J. Chem., 2010, 8(3), 687–695 CrossRef CAS PubMed.
- (a) S. A. Elfeky, S. E. Flower, N. Masumoto, F. D'Hooge, L. Labarthe, W. Chen, C. Len, T. D. James and J. S. Fossey, Chem.–Asian J., 2010, 5, 581–588 CrossRef CAS PubMed; (b) R. Nishiyabu, Y. Kubo, T. D. James and J. S. Fossey, Chem. Commun., 2011, 47, 1106–1123 RSC; (c) R. Nishiyabu, Y. Kubo, T. D. James and J. S. Fossey, Chem. Commun., 2011, 47, 1124–1150 RSC.
- J. Kecht, A. Schlossbauer and T. Bein, Chem. Mater., 2008, 20, 7207–7214 CrossRef CAS.
- (a) J. E. Lofgreen, I. L. Moudrakovski and G. A. Ozin, ACS Nano, 2011, 5(3), 9788–9798 CrossRef PubMed; (b) Y. Gao, Y. Chen, X. Ji, X. He, Q. Yin, Z. Zhang, J. Shi and Y. Li, ACS Nano, 2011, 5(12), 9788–9798 CrossRef CAS PubMed; (c) J. A. Melero, G. D. Stucky, R. van Grieken and G. Morales, J. Mater. Chem., 2002, 12, 1664–1670 RSC.
- S. Xu, L. Chen, J. Li, W. Qina and J. Ma, J. Mater. Chem., 2011, 21, 12047–12053 RSC.
- J. W. Tomsho and S. J. Benkovic, J. Org. Chem., 2012, 77, 2098–2106 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section. See DOI: 10.1039/c4ra16698a |
| This journal is © The Royal Society of Chemistry 2015 |