Potent selective inhibition of MMP-14 by chloroauric acid and its inhibitory effect on cancer cell invasion
Yanyan Wanga,
Hezhen Lua,
Dahai Yua,
Jinrui Zhanga,
Weiguo Lianga,
Zhimin Zhang*b and
Xuexun Fang*a
aKey Laboratory for Molecular Enzymology and Engineering of Ministry of Education, College of Life Science, Jilin University, 2699 Qianjin Street, Changchun, 130012, P. R. China. E-mail: fangxx@jlu.edu.cn; Fax: +86-431-85155200; Tel: +86-431-85155249
bDepartment of Endodontics and Operative Dentistry, School of Stomatology, Jilin University, Changchun, 130021, P. R. China
First published on 2nd February 2015
Abstract
Matrix metalloproteinases (MMPs) are a family of zinc-containing proteases with vital roles in extracellular matrix remodeling. The regulation of MMPs can modulate a number of cellular activities. The therapeutic potential of MMP inhibitors has been shown for diseases such as arthritis and cancer. This paper is the first to demonstrate that HAuCl4 inhibits the activity of MMP-2 and MMP-13 as well as having a more specific inhibitory effect on MMP-14. The effect of HAuCl4 on MMP-14 involves a non-competitive reversible inhibitory mechanism. Moreover, 0–50 μM HAuCl4 did not affect the cell viability of HT-1080 human fibrosarcoma cells. However, HAuCl4 at these concentrations showed significant inhibitory effects on the invasion of the HT-1080 cells, thereby suggesting that HAuCl4 may modulate tumor cell behaviors by inhibiting MMP-14. These findings provide initial clues to further elucidate the biological activity of HAuCl4 and its potential therapeutic value for related diseases.
1 Introduction
Matrix metalloproteinases (MMPs) are a group of zinc-containing proteases that are mostly responsible for extracellular matrix (ECM) turnover.1 MMPs can also regulate the synthesis and secretion of cytokines, growth factors, hormones, and cellular adhesive molecular receptors.2 Consequently, MMPs are involved in various physiological and pathological processes, including embryo formation,3 organ development,4 wound healing,5 tissue remodeling,5 tumorigenesis,6 cancer progression,7 cardiovascular diseases,8 nervous system disease,9 arthritis,10 and respiratory disease,11 among others. To date, a number of MMP inhibitors have been developed for therapeutic purposes against diseases such as cancer and arthritis.12 However, most MMP inhibitors (MMPIs) are organic compounds; only a few inorganic MMPIs have been reported.13 We previously showed that trivalent aluminum salts have inhibitory effects on MMPs and can inhibit the migration and metastasis of tumors without affecting cell viability.14 However, the inhibitory effects of trivalent Au compounds on MMPs and protease-related diseases have not yet been reported.Except for the zero-valence state of colloidal gold, Au mainly exists in monovalent and trivalent states under physiological conditions. Chloroauric acid is an inorganic compound with a crystalline tetrahydrate that is known to contain H5O2+, AuCl4, and two water molecules.15 The AuCl4− anion has a square planar molecular geometry. The Au–Cl distances are approximately 2.28 Å, and other d8 complexes adopt similar structures, e.g., [PtCl4]2−.15,16 Its release of the proton (H+) gives chloroauric acid acidic characteristics. Chloroauric acid is a strongmonoprotic conjugate acid. Although this acid forms aqueous solutions, such solutions are unstable because of the hydrolysis of the tetrachloridoaurate ion. Thus, Au in this acid can be restored to a low-valence state or even a zero-valence state.17 However, this decomposition route is unlikely because HCl is a strong acid; Cl− ions are highly unlikely to combine with H+ to form HCl in an aqueous solution. Gold that exists in different oxidation states has rich coordination chemistry.18 Therefore, even subtle changes in the structure of these metal complexes can cause dramatic changes in their physicochemical and biological properties. Chloroauric acid is the precursor used in purifying gold by electrolysis19 and in preparing gold nanoparticles (AuNPs).20
Au, Pt, and Ru are homologous metals. Gold and its complexes have demonstrated unique biological and medical properties.21 The Au(III) complex showed selective anti-microbial activity against the Gram-positive bacteria (Bacillus cereus and Staphylococcus aureus) being more toxic than its Au(I) analogue, while the free Au(0) is totally inactive.22 Similarly, information on the use of Au(III) complexes as anti-HIV agents is scarce; only a few reports have been recently published.23,24 Gold complexes, with the anti-malaria drug chloroquine as the lead structure, were tested for their activity against Plasmodium falciparum. The activity of some Au(III) cyclometalated complexes was assayed against mammalian and parasitic cysteine proteases, which are involved in the life cycles of parasites, such as Schistosoma, Plasmodium, Trypanosoma brucei, Trypanosoma cruzi, and Leishmania.25–27
Different types of Au(I) and Au(III) compounds possess effective antitumor activity in vitro and in vivo (in animals).28 Au(III) is isoelectronic with Pt(II), and tetracoordinate Au(III) complexes have the same square-planar geometries as cisplatin; therefore, the anticancer activity of Au(III) compounds has been investigated.29 Previous studies suggested that in contrast to cisplatin, gold complexes target proteins but not DNA.30,31 Au(III) dithiocarbamates show anticancer activity, wherein their primary target is the proteasome.32 Treatment of human breast tumor-bearing nude mice with a Au(III) dithiocarbamate complex causes significant inhibition of tumor growth, which is associated with proteasome inhibition and massive apoptotic induction in vivo.28,32 Gold possesses two beta-emitting radioactive isotopes, namely, 198Au and 199Au, which are potentially suitable for therapeutic applications.33 A number of Schiff base and thiosemicarbazonato complexes are prepared with 198Au.33
To date, the greatest concern is the study of disease treatment with nanometer gold complexes, especially for anticancer treatment.34 The combined physical, chemical, optical, and electronic properties of gold nanoparticles (AuNPs) provide a new platform for imaging and diagnosis of cancer.35–40 Therefore, drugs can be selectively provided,27,41–43 treatment can target sensitive cells and tissues,44,45 surgery can be supervised and guided, and chemotherapy can be specifically given to specific disease loci.46,47 However, other studies also found that nanometer gold can promote the rapid increase of NO in the blood. The released NO inside the cell may react with the superoxide anion (O2−) to generate peroxynitrite anion (ONOO−), which has strong oxidation activity and greater destructive effects, thereby inducing a series of oxidative stress effects. This discovery warns against the application of nanometer gold as biological probes, drug carriers, and vehicles in cells or organisms. Furthermore, Au can affect the activation or nuclear translocation of transcription factors formed by the binding of NF-κB with DNA and can play an important role in the signal pathway.48 An improved understanding of the physiological processes of gold compounds will provide a rational basis for their further development into novel anticancer drugs.
In this paper, we analyzed the inhibition effects of chloroauric acid on the enzyme activity of representative members of the MMP family. MMP-2, -13, and -14 belong to the gelatin enzymes, collagen-type enzymes, and membrane MMPs, respectively.49 Given the specific degradation of different substrates, these MMPs can degrade different types of collagen and gelatin. MMPs participate in multiple physiological or pathological processes that require ECM remodeling, and their excessive expression is closely related to migration, invasion, and metastasis of tumor cell during tumor progression.50–52 The mechanism and type of inhibition were analyzed by enzyme kinetics. Moreover, the influence of chloroauric acid on the cell viability and invasive ability of HT-1080 tumor cells was analyzed at the cytological level. Thus, the inhibition mechanism of chloroauric acid on MMPs was inferred. This study is vital in exploring the significant role of gold compounds in related diseases. The potential value of MMPs for the development of novel relevant drugs was demonstrated.
2 Materials and methods
2.1 Materials and reagents
DQ-gelatin was purchased from Invitrogen. The recombinant catalytic domains of human MMP-2, -13, and -14 were expressed in Escherichia coli, purified, and refolded in our laboratory.53 Gelatin was obtained from Sigma. Other reagents and solvents used in experiments were of analytical or reagent grade, as deemed appropriate.2.2 Enzyme activity assay
An FLx800 fluorescence microplate reader (Bio-Tek) was used to measure the MMP activity. DQ-gelatin was employed as the substrate. Kinetics assays were performed at 37 °C in 50 mM HEPES buffer (pH 7.5) containing 0.2 M NaCl, 1 mM CaCl2, 20 μM ZnSO4, and 0.05% Brij-35. For the inhibition assays, metal ions were incubated with an appropriate quantity of MMPs for 15 min to ensure that equilibrium was reached before adding the fluorescent substrate. The extent of inhibition was determined using the initial rates with and without the inhibitor.54 The FLx800 fluorescence microplate reader (Bio-Tek) was also used to measure the cathepsin activity, with z-Phe-Arg-AMC as the substrate. Cathepsin B: 25 mM MEs, 5 mM DTT, pH 6.0; Cathepsin L: 0.4 M NaOAc, 4 mM EDTA, 8 mM DTT, pH 5.5. For the enzyme inhibition assays, the activity data were fitted in a linearised curve using Origin version 7.5. The IC50 values were then determined from the curve accordingly.2.3 Enzyme kinetics of chloroauric acid inhibition on MMP-14
To determine the inhibitory mechanism, we performed further kinetic analysis of the inhibition of chloroauric acid on MMP-14. Different quantities of enzyme (0.125–1 μg mL−1) were added into the reaction mixture, and then the same substrate and inhibitor concentration (0.2 and 0.4 μM) were added. Under different concentrations of the enzyme and inhibitor, the reaction rate was reduced with gradient concentration-dependence as compared with the control group. All straight lines in the standardization plot passed through the zero point. Therefore, the inhibition of MMP-14 by chloroauric acid was initially determined as reversible inhibition.To analyze the inhibitory type, we selected 1 μg mL−1 as the final concentration of the enzyme. The final concentrations of the inhibitor were selected as 0, 0.2, 0.4, and 0.6 μM, respectively, according to the detected IC50 = 0.3 μM during the inhibition of MMP-14 by chloroauric acid; substrates with different final concentrations (0.5–5 μg mL−1) were added. The inhibition type was obtained by the Lineweaver–Burk and Dixon plots.55,56
2.4 Cell culture
The human fibrosarcoma carcinoma cell line HT-1080 was cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) supplemented with penicillin (100 units per mL) and streptomycin (100 μg mL−1) at 37 °C with 5% CO2.2.5 Measurement of cell viability (MTT)
HT-1080 cells were seeded into 96-well plates at a density of 1 × 104 cells per well in 200 μL of DMEM containing 10% fetal bovine serum. At 24 h after seeding, the medium was removed, and the cells were incubated for 24 h with DMEM containing 10% FBS in the absence or presence of various concentrations of metal ions. Subsequently, 200 μL of DMEM and 20 μL of 5 mg mL−1 MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) solution was added to each well. The plates were placed in an incubator at 37 °C in 5% CO2 for 4 h. The medium was then replaced with 150 μL of DMSO, and the absorbance was recorded at 490 nm.2.6 Matrigel invasion assay
The Boyden chamber technique (transwell analysis) was performed as previously described.57 Cancer cells were treated with chloroauric acid or solvent (as control). Homogeneous single-cell suspensions (1 × 105 cells per well) were added to the upper chambers. The cells were allowed to invade the wells at 37 °C for 24 h in a CO2 incubator. Migrated cells were stained with 0.1% crystal violet at room temperature for 10 min and examined by light microscopy. Quantification of migrated cells was performed according to published criteria.3 Results and discussion
3.1 Chloroauric acid specifically suppressed the activity of MMP-14 in a dose-dependent manner
Our previous work on trivalent Al on MMP-16 revealed that these trivalent metal salts and their complexes all have significant effects on the activity of MMP-16 as well as the migratory and invasive abilities of the cells.14 The significant inhibition of MMPs by these inorganic trivalent metal salts is a new discovery in the field of MMP inhibitors. Chloroauric acid is a trivalent compound of gold. Thus, MMP-2, -13, and -14 were selected in our study to analyze whether chloroauric acid has inhibitory effects on MMPs. The average values of the three measurements are shown in Fig. 1a–c. The results showed that with the increasing concentration, chloroauric acid had significant inhibitory effects on MMP-2, -13, and -14, with IC50 values in the micromolar and sub-micromolar range. The inhibition of MMP-14 was particularly remarkable. Control experiment with fluorogenic substrate in presence of HAuCl4 alone were performed in the absence of the MMPs (data not shown).To analyze whether chloroauric acid can specifically inhibit MMPs, we selected the members of cathepsin family (Cathepsin B and L) with different catalytic mechanisms from MMP family for the enzyme activity analysis. Cathepsins belong to the cysteine protease family. These enzymes have a cysteine in their catalytic sites that starts the proteolysis cascade reactions. Cathepsins have an important role in promoting the occurrence and development of tumors. Among the cathepsins, Cathepsin B can promote the tumor progression through the degradation of the basement membrane as well as the ECM components and elements.58 In addition, the proliferation and growth of tumor cells with the silenced Cathepsin L gene are slower and the invasion is significantly reduced.59 Chloroauric acid at a final concentration of 10 μM was found to have no obvious inhibitory effects on the two cathepsins (Table 1). A comparison of enzyme inhibition by chloroauric acid on three recombinant proteases (MMP-2, -13, and -14) and cathepsins (Cathepsin B and L) implies that chloroauric acid has inhibitory effects on three MMPs, with relative specificity for MMP-14 (Table 1).
| Proteinases | IC50 (μM) | ||
|---|---|---|---|
| HAuCl4 | NaAuCl4 | NaCl | |
| a Note: no means no inhibition were observed to the activity of the corresponding enzyme with 10 μM metal complex. | |||
| MMP-2 | 2.28 | 6 | No |
| MMP-13 | 9.83 | 8 | No |
| MMP-14 | 0.30 | 10 | No |
| Cathepsin B | No | No | No |
| Cathepsin L | No | No | No |
3.2 Inhibition mechanism and types of chloroauric acid on MMP-14
Chloroauric acid possesses significant inhibitory effects on MMP-2, -13, and -14, especially for MMP-14. By contrast, the effects on Cathepsin B and L are not significant. Therefore, the inhibition of chloroauric acid on MMP-14 is specific to a certain degree although the specific inhibition mechanism remains unclear. To evaluate the mechanism and type of inhibition, we performed further dynamic analysis on the inhibition of MMP-14 by chloroauric acid. Different enzyme concentrations were added into the test system, and then the same amount of substrate and inhibitor was added. Changes in the enzyme activity were determined, and the curves of enzyme concentration and enzyme activity were drawn (Fig. 2a). Under different concentrations of the enzyme (0.125–1 μg mL−1) and inhibitor (0.2 and 0.4 μM), the reaction rate was reduced as compared with the control group, with a gradient concentration dependent. All the straight lines in the plot passed through the zero point; therefore, the inhibition of MMP-14 by chloroauric acid was initially determined as reversible inhibition.In the following experiments, 1 μg mL−1 was selected as the final concentration of enzyme to analyze the inhibitory type; the respective final concentrations of inhibitors were 0, 0.2, 0.4, and 0.6 μM, according to the detected IC50 = 0.3 μM for the inhibition of MMP-14 by chloroauric acid; substrates with different final concentrations (0.5–5 μg mL−1) were then added. A plot of the Michaelis–Menten equation is presented in Fig. 2b. Km remained constant whereas Vmax decreased with increasing inhibitor concentration. The plot of the Lineweaver–Burk equation is shown in Fig. 2c. With increasing concentration, Km was constant but Vmax decreased. Therefore, the observed inhibition is non-competitive. The Dixon plot equation was used to obtain Fig. 2d. The two straight lines (representing substrate concentrations of 1 and 2 μg mL−1) intersected at the second quadrant, with the position of intersection point as −Ki, which suggests non-competitive inhibition. Therefore, chloroauric acid noncompetitively inhibited MMP-14 within the concentration range of 0–0.6 μM. That is, a competitive relationship does not exist between the respective effects of the substrate and chloroauric acid on MMP-14. The combination of MMP-14 with the substrate or chloroauric acid does not influence other combinations, but the formed compound cannot be degraded, thereby resulting in decreased enzyme activity. The binding site is the group beyond the active site. Its structure is not similar to that of the substrate, and the inhibition cannot be relieved by increasing the concentration of substrate.
A number of studies based on different physicochemical techniques suggest that the probable binding sites of Au(III) are N(1)/N(7) atoms of adenosine, N(7) or C(6)O of guanosine, N(3) of cytidine, and N(3) of thymidine, which are analogous to the possible binding sites of the isoelectronic Pt(II) ion.60 Recent in vitro studies showed that the interactions of several Au(III) complexes with calf thymus DNA are weak, whereas significant binding to model proteins occurs.61 This phenomenon is supported by Fricker et al.,30 who demonstrated that a Au(III)–damp complex has a clear preference for S-donor ligands, such as glutathione and cysteine, with only limited reactivity against nucleosides and their bases. Therefore, a novel mechanism was proposed, wherein proteins containing exposed cysteine residues may be proper targets for that class of Au(III) complexes. Au(III) complexes also interact with bovine serum albumin.62 These complexes make very stable adducts; once these adducts are formed, they are destroyed only by adding strong ligands for Au(III), such as cyanide.63 Based on these findings, the selective modification of surface protein residues by Au(III) compounds could be the molecular basis for their biological effects. This hypothesis has prompted the search for novel gold–protein interactions in an attempt to identify possible targets responsible for the biological effects of gold compounds. The key proteins that are modified by Au(III) complexes and responsible for triggering apoptosis have yet to be identified.64
MMP-14 is synthesized with a signal peptide (Signal), a prodomain (Pro) for latency, a catalytic domain (Catalytic) with catalytic zinc ion (Zn) for proteolytic activity, a linker-1 (L1), a hemopexin domain (Hpx), a linker-2 (L2), a transmembrane domain (TM), and a cytoplasmic tail (CP).65 Our results indicated that inhibition by chloroauric acid is achieved by interaction with a site other than the active center of MMP-14 within the catalytic domain of the enzyme. However, the exact location of the interaction remains to be identified.
3.3 Toxicity of chloroauric acid on HT-1080 cells by MTT assay
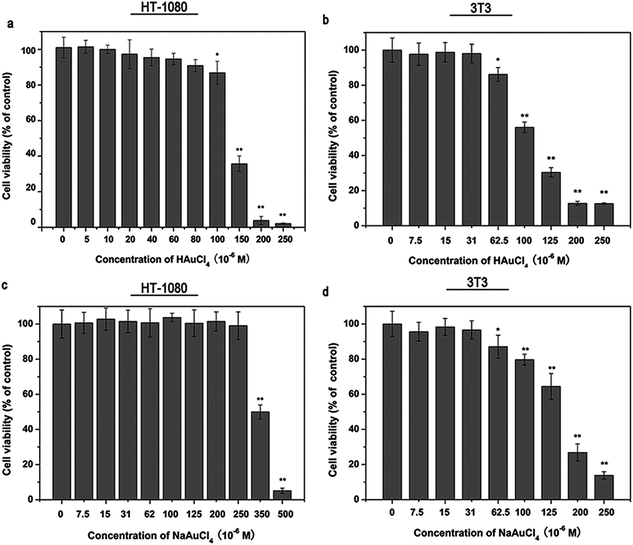
Based on the specific inhibitory effects of chloroauric acid on MMPs, especially MMP-14, the influence of chloroauric acid on the behavior of MMP-14-related tumor cells was analyzed. The MTT assay was used to determine the toxicity of chloroauric acid on HT-1080 cells. As shown in Fig. 3a, chloroauric acid has certain influence to the viability of HT-1080 cells. Its IC50 was approximately 125 μM. No evident effects on the cell viability were observed when the chloroauric acid concentration was less than 100 μM. When the chloroauric acid concentration was less than 100 μM, increased cytotoxicity was notably absent despite the increasing concentration. However, the cell viability suddenly decreased when its concentration was greater than 100 μM. This trend may be attributed to the sudden increase of cell permeability when chloroauric acid enters into the cells, which eventually leads to cell death and decreased cell viability. According to the aforementioned results, 0–50 μM was selected as the drug concentration range in the subsequent cell experiments to study its influence on the behavior of the tumor cells without affecting the cell vitality. We also performed the cell viability test of chloroauric acid and its sodium salt on a non-cancerous cell line, 3T3 cells, a established mouse embryo fibroblast cell line. The result is shown in Fig. 3b. Our result revealed that chloroauric acid exhibited similar effect on the viability of HT-1080 cells and 3T3 cells. NaAuCl4 showed similar effect on the viability of HT1080 cells and 3T3 cells compare to the chloroauric acid (Fig. 3c and d). However, it reduced the cell viability of HT-1080 cells at a much higher concentration compare to the chloroauric acid.3.4 Chloroauric acid can effectively inhibit the invasion behaviors of HT-1080 cells
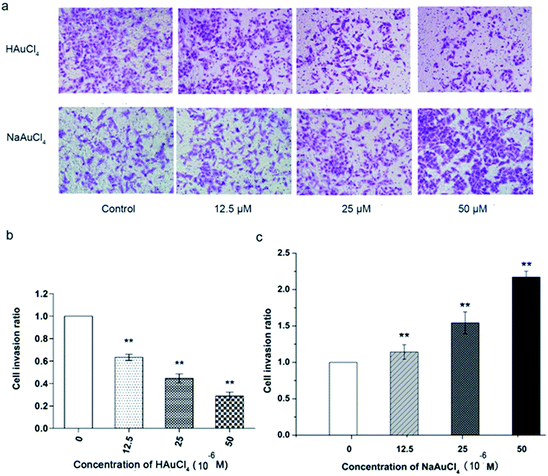
The Matrigel invasion assay is a common method to study tumor cells. Given their invasive nature, tumor cells can degrade matrix by secreting hydrolytic enzymes such as MMPs, thereby entering into the lower culture chamber through the micropores and then adhering to the bottom of the chamber. After staining, the invasive ability of tumor cells could be analyzed. An invasion assay was used to analyze the influences of chloroauric acid on the invasive ability of HT-1080 under conditions that do not affect the cell vitality. As shown in Fig. 4a and b, the number of invasion cells was significantly decreased with increasing drug concentration. When the final concentration was 25 μM, the invasion rate was <50% as compared with the control group. When the final concentration of chloroauric acid was 50 μM, the invasion rate dropped to approximately 35%. Therefore, the aforementioned results show that chloroauric acid has obvious inhibitory effects on the invasion of HT-1080 cells, and this inhibition is concentration dependent. As MMP-14 is a key enzyme in controlling the focal degradation of ECM during cell migration,66 it is reasonable to speculate that chloroauric acid inhibits MMP-14 activity in the cell membrane surface and inhibits the ability of degradation of extracellular matrix, which affect the cell migration and invasion.Chloroauric acid has a specific inhibition effect on MMP-14 and can effectively inhibit the invasion behaviors of HT-1080 cells. Further measurement of the pH value of cell culture media containing the compound and the enzyme activity measurement system revealed that the pH value of the reaction system is not changed in the applied concentrations of chloroauric acid. Therefore, the inhibition of MMP-14 chloroauric acid, as well as the influence of chloroauric acid on cell migration and invasion, was not influenced by the changing system pH. To analyze whether AuCl4− is the active group in the influences of chloroauric acid on HT-1080 cells, we selected the relatively stable sodium salt of chloroauric acid for an enzymology study to determine the effects on the behavior of tumor cells. The results show that both compounds had different inhibitory effects on proteases. NaAuCl4·2H2O exhibited an inhibitory effect on the MMP-2, -13, and -14 and did not suppress cathepsins, but its inhibition of MMP-2, -13, and -14 was weaker than that of chloroauric acid, especially for MMP-14, with IC50 values of 8, 6, and 10 μM, respectively (Table 1). In addition, its effects on the cytotoxicity and behavior of HT-1080 cells were compared. The MTT assay (Fig. 3c) showed that NaAuCl4 had certain influence to the viability of HT-1080 cells, with an IC50 of approximately 350 μM. When NaAuCl4 was less than 250 μM, no evident effects on cell viability were observed. Compared with chloroauric acid (Fig. 3a and b), NaAuCl4 had lower toxicity in HT-1080 cells (Fig. 3c) and 3T3 cells (Fig. 3d). Under conditions that did not affect cell vitality, NaAuCl4 (12.5–50 μM) had no obvious inhibitory effects on the migration and invasion of HT-1080 cells. On the contrary, results showed that NaAuCl4 promoted these behaviors, as shown in Fig. 4a and c. The mechanism of this phenomena is unclear. Sodium ions are involved in various biological processes and are significant for the maintenance of cell vitality, which may eventually leads to the very different effects of the two compounds on tumor cell behaviors. Although we attempted to analyze the inhibitory effects of both compounds, further studies are still needed to clarify the effects of these two compounds on cell vitality.
In addition, based on the great structural variety of the ligands and the derived complexes, a unique mode of action or pharmacological profile for all Au(III) complexes is unlikely to exist. In particular, direct DNA damage, modification of the cell cycle, mitochondrial damage, such as thioredoxin reductase (TrxR) inhibition, proteasome inhibition, modulation of specific kinases, and other cellular processes, are affected by gold compounds; their interaction eventually triggers apoptosis, which seems to have a major role in the mechanism of action of gold compounds.21,28 Research on these topics is significant in understanding the roles of Au(III) and Au complexes in regulating the activity of MMPs.
4 Conclusions
In this paper, the results show that chloroauric acid have specific inhibitions to MMP-14, and the inhibition mechanisms are the non-competitive type, namely there is no competitive relationship between the effects of the substrate and chloroauric acid on MMP-14. These results indicate that the inhibition are performed by combining the compounds with the non-active center of MMP-14 and changing its structure and conformation, which has not been reported so far. We have also found that chloroauric acid can affect the behaviors of tumor cell HT-1080, and in particular, chloroauric acid may play a role in tumor cell behaviors by inhibiting MMP-14. The research of thesis is very meaningful for the understanding of Au(III) and Au complexes in regulating the activity of MMPs.Acknowledgements
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (no. 31170742, 31100574 and 31370742).References
- I. H. Bellayr, X. Mu and Y. Li, Future Med. Chem., 2009, 1, 1095–1111 CrossRef CAS PubMed.
- V. Vargová, M. Pytliak and V. Mechírová, EXS, 2012, 103, 1–33 Search PubMed.
- P. Bischof and A. Campana, Baillieres Best Pract. Res. Clin. Obstet. Gynaecol, 2000, 14, 801–814 CrossRef CAS PubMed.
- V. Srinivasan, D. W. Spence, A. Moscovitch, S. R. Pandi-Perumal, I. Trakht, G. M. Brown and C. D. P. Cardinali, J. Pineal Res., 2010, 48, 1–8 CrossRef CAS PubMed.
- K. Ganguly, A. V. Sharma, R. J. Reiter and S. Swarnakar, J. Pineal Res., 2010, 49, 130–140 CAS.
- M. D. Sternlicht and Z. Werb, Annu. Rev. Cell Dev. Biol., 2001, 17463–17516 Search PubMed.
- M. Ege blad and Z. Werb, Nat. Rev. Cancer, 2002, 2, 161–174 CrossRef CAS PubMed.
- J. F. Dilmé, S. Bellmunt, M. Camacho, D. Solà-Villà, J. M. Romero, J. R. Escudero and L. Vila, Eur. J. Vasc. Endovasc. Surg., 2014, 48, 374–381 CrossRef PubMed.
- J. M. Milner and T. E. Cawston, Curr. Drug Targets: Inflammation Allergy, 2005, 4, 363–375 CrossRef CAS.
- P. S. Burrage and C. E. Brinckerhoff, Curr. Drug Targets, 2007, 8, 293–303 CrossRef CAS.
- G. Murphy and H. Nagase, Nat. Clin. Pract. Rheumatol., 2008, 4, 128–135 CrossRef CAS PubMed.
- G. Murphy and H. Nagase, Mol. Aspects Med., 2008, 29, 290–308 CrossRef CAS PubMed.
- A. K. Chaudhary, S. Pandya, K. Ghosh and A. Nadkarni, Mutat. Res., 2013, 753, 7–23 CAS.
- Y. Shen, S. Liu, F. Jin, T. Mu, C. Li, K. Jiang, W. Tian, D. Yu, Y. Zhang and X. Fang, BioMetals, 2012, 25, 541–551 CrossRef CAS PubMed.
- J. M. Williams and S. W. Peterson, J. Am. Chem. Soc., 1969, 91, 776–777, DOI:10.1021/ja01031a062 , ISSN 0002-7863.
- A. J. Bridgeman, Inorg. Chem., 2008, 47, 4817–4825 CrossRef CAS PubMed.
- L. Ronconi, L. Giovagnini, C. Marzano, F. Bettio, R. Graziani, G. Pilloni and D. Fregona, Inorg. Chem., 2005, 44, 1867–1881 CrossRef CAS PubMed.
- P. J. Sadler, J. Rheumatol., Suppl., 1982, 8, 71–78 CAS.
- Y. Lei, H. Chen, H. Dai, Z. Zeng, Y. Lin, F. Zhou and D. Pang, Biosens. Bioelectron., 2008, 23, 1200–1207 CrossRef CAS PubMed.
- O. Smithies, M. Lawrence, A. Testen, L. P. Horne, J. Wilder, M. Altenburg, B. Bleasdale, N. Maeda and T. Koklic, Langmuir, 2014, 30, 13394–13404 CrossRef CAS PubMed.
- P. I. Maia, V. M. Deflon and U. Abram, Future Med. Chem., 2014, 6, 1515–1536 CrossRef CAS PubMed.
- A. Nakajima, World J. Microbiol. Biotechnol., 2003, 19, 369–374 CrossRef CAS.
- Metallotherapeutic Drugs and Metal-Based Diagnostic Agents: The Use of Metals in Medicine, ed. M. Gielen and E. R. T. Tiekink, John Wiley, Sons, West Sussex, UK, 2005 Search PubMed.
- F. Liu, R. Anis, E. Hwang, R. Ovalle, A. Varela-Ramírez, R. J. Aguilera and M. Contel, Molecules, 2011, 16, 6701–6720 CrossRef CAS PubMed.
- P. I. Maia, A. G. Fernandes, J. J. Silva, A. D. Andricopulo, S. S. Lemos, E. S. Lang, U. Abram and V. M. Deflon, J. Inorg. Biochem., 2010, 104, 1276–1282 CrossRef CAS PubMed.
- M. Navarro, Coord. Chem. Rev., 2009, 253, 1619–1626 CrossRef CAS PubMed.
- M. Navarro, F. Vasquez, R. A. Sanchez-Delgado, H. Perez, V. Sinou and J. Schrevel, J. Med. Chem., 2004, 47, 5204–5209 CrossRef CAS PubMed.
- V. Milacic, D. Fregona and Q. P. Dou, Histol. Histopathol., 2008, 23, 101–108 CAS.
- M. J. McKeage, L. Maharaj and S. J. Berners-Price, Coord. Chem. Rev., 2002, 232, 127–135 CrossRef CAS.
- S. P. Fricker, R. Skerjl, B. R. Cameron, R. Mosi and Y. Zhu, Recent developments in gold drugs 2003, AnorMED Inc, Langley BC, V2Y 1N5, Canada, GOLD, 2003 Search PubMed.
- G. Marcon, L. Messori, P. Orioli, M. A. Cinellu and G. Minghetti, Eur. J. Biochem., 2003, 270, 4655–4661 CrossRef CAS.
- V. Milacic, D. Chen, L. Ronconi, K. R. Landis-Piwowar, D. Fregona and Q. P. Dou, Cancer Res., 2006, 66, 10478–10486 CrossRef CAS PubMed.
- B. N. Bottenus, P. Kan, T. Jenkins, B. Ballard, T. L. Rold, C. Barnes, C. Cutler, T. J. Hoffman, M. A. Green and S. S. Jurisson, Nucl. Med. Biol., 2010, 37, 41–49 CrossRef CAS PubMed.
- C. M. Che and R. W. Sun, Chem. Commun., 2011, 47, 9554–9560 RSC.
- E. C. Dreaden, L. A. Austin, M. A. Mackey and M. A. El-Sayed, Ther. Delivery, 2012, 3, 457–478 CrossRef CAS.
- H. Wang, T. B. Huff, D. A. Zweifel, W. He, P. S. Low, A. Wei and J. X. Cheng, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 15752–15756 CrossRef CAS PubMed.
- C. L. Zavaleta, B. R. Smith, I. Walton, W. Doering, G. Davis, B. Shojaei, M. J. Natan and S. S. Gambhir, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 13511–13516 CrossRef CAS PubMed.
- W. Lu, Q. Huang, G. Ku, X. Wen, M. Zhou, D. Guzatov, P. Brecht, R. Su, A. Oraevsky, L. V. Wang and C. Li, Biomaterials, 2010, 31, 2617–2626 CrossRef CAS PubMed.
- D. Kim, Y. Y. Jeong and S. Jon, ACS Nano, 2010, 4, 3689–3696 CrossRef CAS PubMed.
- G. Von Maltzahn, A. Centrone, J. H. Park, R. Ramanathan, M. J. Sailor, T. A. Hatton and S. N. Bhatia, Adv. Mater., 2009, 21, 1–6 CrossRef PubMed.
- E. C. Dreaden, S. C. Mwakwari, Q. H. Sodji, A. K. Oyelere and M. A. El-Sayed, Bioconjugate Chem., 2009, 20, 2247–2253 CrossRef CAS PubMed.
- D. A. Giljohann, D. S. Seferos, A. E. Prigodich, P. C. Patel and C. A. Mirkin, J. Am. Chem. Soc., 2009, 131, 2072–2073 CrossRef CAS PubMed.
- S. Dhar, W. L. Daniel, D. A. Giljohann, C. A. Mirkin and S. J. Lippard, J. Am. Chem. Soc., 2009, 131, 14652–14653 CrossRef CAS PubMed.
- J. F. Hainfeld, F. A. Dilmanian, Z. Zhong, D. N. Slatkin, J. A. Kalef-Ezra and H. M. Smilowitz, Phys. Med. Biol., 2010, 55, 3045–3046 CrossRef CAS PubMed.
- D. B. Chithrani, S. Jelveh, F. Jalali, M. van Prooijen, C. Allen, R. G. Bristow, R. P. Hill and D. A. Jaffray, Radiat. Res., 2010, 173, 719–728 CrossRef CAS PubMed.
- X. M. Qian, X. H. Peng, D. O. Ansari, Q. Yin-Goen, G. Z. Chen, D. M. Shin, L. Yang, A. N. Young, M. D. Wang and S. Nie, Nat. Biotechnol., 2008, 26, 83–90 CrossRef CAS PubMed.
- Y. Jung, R. Reif, Y. Zeng and R. K. Wang, Nano Lett., 2011, 11, 2938–2943 CrossRef CAS PubMed.
- J. B. Lewis, J. C. Wataha, V. McCloud, P. E. Lockwood, R. L. Messer and W. Y. Tseng, J. Biomed. Mater. Res., Part A, 2005, 74, 474–481 CrossRef CAS PubMed.
- C. Amălinei, I. D. Căruntu and R. A. Bălan, Rom. J. Morphol. Embryol., 2007, 48, 323–334 Search PubMed.
- G. Ardif, P. Reboul, J. P. Pelletier and J. Martel-Pelletier, Mod. Rheumatol., 2004, 14, 197–204 CrossRef PubMed.
- K. Hashizume, J. Reprod. Dev., 2007, 53, 1–11 CrossRef CAS.
- M. A. Weaver, Clin. Exp. Metastasis, 2006, 23, 97–105 CrossRef PubMed.
- X. Shi, F. Jin, H. Wang, J. Yang, Z. Wang and X. Fang, Chem. Res. Chin. Univ., 2006, 22, 129–133 CrossRef CAS.
- J. Yang, Y. Shen, Y. Hong, F. Jin, S. Zhao, M. Wang, X. Shi and X. Fang, J. Ethnopharmacol., 2008, 117, 285–289 CrossRef PubMed.
- X. Fang, Y. Zhan, J. Yang and D. Yu, J. Mol. Catal. B: Enzym., 2014, 104, 1–7 CrossRef CAS PubMed.
- X. Zhang, L. Shi, X. Li, Q. Sheng, L. Yao, D. Shen, Z. R. Lü, H. M. Zhou, Y. D. Park, J. Lee and Q. Zhang, J. Biosci. Bioeng., 2014, 117, 696–705 CrossRef CAS PubMed.
- H. Ji, J. Wang, B. Fang, X. Fang and Z. Lu, J. Neuro-Oncol., 2011, 103, 445–451 CrossRef CAS PubMed.
- J. L. Johnson, S. J. George, A. C. Newby and C. L. Jackson, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 15575–15580 CrossRef CAS PubMed.
- Y. S. Kim and T. H. Joh, Biomol. Ther., 2012, 20, 133–143 CrossRef CAS PubMed.
- S. T. Crooke and C. K. Mirabelli, Am. J. Med., 1983, 75, 109–113 CrossRef CAS.
- C. Marzano, F. Bettio, F. Baccichetti, A. Trevisan, L. Giovagnini and D. Fregona, Chem.–Biol. Interact., 2004, 148, 37–48 CrossRef CAS PubMed.
- X. M. He and D. C. Carter, Nature, 1992, 358, 209–215 CrossRef CAS PubMed.
- L. Messori, G. Marcon and P. Orioli, Bioinorg. Chem. Appl., 2003, 1, 177–187 CrossRef CAS PubMed.
- K. H. Chow, R. W. Sun, J. B. Lam, C. K. Li, A. Xu, D. L. Ma, R. Abagyan, Y. Wang and C. M. Che, Cancer Res., 2010, 70, 329–337 CrossRef CAS PubMed.
- Y. Itoh, IUBMB Life, 2006, 58, 589–596 CrossRef CAS PubMed.
- A. V. Chernov, N. Eddine Sounni, A. G. Remacle and A. Y. Strongin, J. Biol. Chem., 2009, 284, 12727–12734 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |