A polymer supported ascorbate functionalized task specific ionic liquid: an efficient reusable catalyst for 1,3-dipolar cycloaddition†
Jayavant D. Patila,
Supriya A. Patilb and
Dattaprasad M. Pore*a
aDepartment of Chemistry, Shivaji University, Kolhapur 416 004, India. E-mail: p_dattaprasad@rediffmail.com; Fax: +91 231 2692333; Tel: +91 231 2690571
bDepartment of Chemistry, Hanyang University, Sungdong-Ku, Haengdang-dong 17, Seoul, 133-791, Republic of Korea
First published on 17th February 2015
Abstract
A novel polymer supported ascorbate functionalized task specific ionic liquid was designed and synthesized. It has been demonstrated that the synthesized polymer supported ionic liquid acts as an efficient catalyst for regioselective Huisgen 1,3-dipolar cycloaddition of aryldiazonium tetrafluoroborate, sodium azide and terminal alkyne, which provide a series of 1,4-disubstituted-1,2,3-triazoles in high yields at room temperature as well as showing an aptitude for in situ incorporation of copper.
Introduction
The increasing environmental consciousness of the chemical community has led to the search for more efficient methods for organic synthesis acquiring the principles of Green chemistry.1 This goal was achieved by employing green catalysts/solvents (e.g., water, ionic liquids) as well as efficient and cleanly reusable catalytic materials.2 Ionic liquids have emerged as ecofriendly catalysts as well as multi-use reaction media and green solvent for a variety of applications due to the fact that they possess negligibly small vapor pressure, high thermal, chemical as well as electrochemical stability with widely tunable properties with regard to polarity, hydrophobicity, solvent miscibility, etc.3 Despite these advantages, a series of drawbacks such as their high cost as compared to traditional catalysts and solvents, low biodegradability and (eco)toxicological properties still exists,4 their recovery requires tedious process viz. distillation.5 To circumvent the limitations raised with ILs, the use of supported ionic liquid phase (SILP) catalysts has been devised which possess advantages of ionic liquid media with solid support materials, enables the application of fixed-bed technology and the usage of significantly reduced amounts of the ionic liquid. It involve immobilization of ILs onto a surface of a porous high area support material.6 A number of benefits arise from the use of SILP catalysts, some of which are the ease of separation of catalyst, simply by filtration, recycling and reproducibility as well as facilitating significant advances in selectivity. Due to these reasons, in last few years SILP catalysts have attracted attention of scientists and due to its influence several organic transformations have been successfully accomplished.71,2,3-Triazoles are an important class of heterocyclic compounds, the interest in their synthesis stems from their wide range of applications in pharmaceuticals, agro chemicals, dyes, photographic materials, corrosion inhibition,8 etc. and biological activities such as antiviral, antiepileptic, antiallergic,9 anticancer,10 anti-HIV,11 antimicrobial activities against gram positive bacteria and b3-adrenergic receptor agonist.12 Due to such multifarious applications different methods have been exploited for their synthesis13 and amongst them Huisgen 1,3-dipolar cycloaddition of azides with alkynes is the most popular method for the construction of 1,2,3-triazole framework.14
However, the early Huisgen 1,3-dipolar cycloaddition15a–c process required a strong electron-withdrawing substituent either on azide or on alkyne, and were often conducted at high temperature for a prolonged period of time, and usually led to the isolation of a mixture of 1,4-disubstituted- and 1,5-disubstituted-1,2,3-triazoles.15d,e
Sharpless et al. used Cu(I) salts to promote the reaction of azide with terminal alkynes to afford 1,4-disubstituted products.16 Fokin and co-workers have developed the method employing microwave irradiations.17 Moreover in recent years, several methods have been reported for the synthesis of triazoles18 generally through the coupling reaction between alkynes and azides at high temperatures19 and by using solid supported catalysts20 or nanocatalysts.21 Many of the literature methods are plagued by disadvantages such as use of organic solvent, no reusability of catalytic system, long reflux time, influence of microwave irradiation, organic azide as reagent and limited substrate scope. Thus, the development of an efficient, eco-friendly, regioselective protocol for Huisgen 1,3-dipolar cycloaddition having wide substrate scope operable at ambient temperature is highly warranted. To achieve this goal, we described synthesis of novel polymer supported ascorbate functionalized task specific ionic liquid [MR-IMZ-As] for synthesis of 1,4-disubstituted 1,2,3-triazoles employing click approach (Scheme 1).
 | ||
| Scheme 1 Polymer supported ascorbate functionalized task specific ionic liquid (MR-IMZ-As) catalyzed Huisgen 1,3-dipolar cycloaddition. | ||
Result and discussion
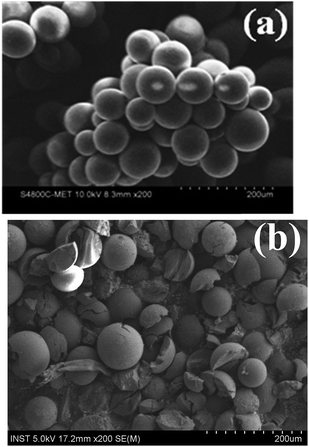
Initial attempts were focused on synthesis of novel polymer supported ionic liquid, which is ecofriendly, recyclable, having capacity to stabilize copper and cost effective for Huisgen 1,3-dipolar cycloaddition (Fig. 1).In this context, we designed MR-IMZ-As Merrifield resin (polymer) supported ascorbate functionalized task specific ionic liquid. Initially, polymer supported ionic liquid 3 was prepared by treating Merrifield resin with 1-methyl imidazole in toluene employing reflux conditions for 24 h. This on anion exchange using potassium hydroxide in water for 12 h at room temperature furnished 4. Formation of 3 and 4 was characterized by infrared spectroscopy. Finally, reacting suspension of 4 with a solution of ascorbic acid in water at room temperature for 12 h led polymer supported ascorbate functionalized task specific ionic liquid 6 (Scheme 2), which was characterized using IR, SEM and EDX analysis.
 | ||
| Scheme 2 Preparation of polymer supported ascorbate functionalized task specific ionic liquid (MR-IMZ-As). | ||
IR spectrum (Fig. 2) of MR-IMZ-As (C) shows, absorption band at 1720 cm−1 corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of lactone and at 1626, 1602, 1562 and 1158 cm−1 corresponding to C
O stretching of lactone and at 1626, 1602, 1562 and 1158 cm−1 corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C–N and C–O stretching, respectively. The SEM images of MR-IMZ-As before and after use for present transformation exhibit the morphological differences (Fig. 3).
N, C–N and C–O stretching, respectively. The SEM images of MR-IMZ-As before and after use for present transformation exhibit the morphological differences (Fig. 3).
Next, we focused our attention to explore this novel polymer supported ascorbate functionalized task specific ionic liquid (MR-IMZ-As) in the 1,3-dipolar cycloaddition. Aromatic azides are generally used as one of the precursor in Huisgen 1,3-dipolar cycloaddition, they are also versatile intermediates in several organic reactions22 viz. Ugi, Griess, Staudinger, Curtius and Schmidt, etc. Although, these organic azides are stable against most reaction conditions, low molecular weight azides are explosive, high temperatures lead to decomposition of the organic azides.23 To overcome the drawbacks raised due to organic azides as direct precursors, scientific community preferred in situ generation of organic azides to achieve a desired transformation.
Aryl diazonium salts are best ecofriendly alternative to organic azides as it easily generates aryl azide by reaction with sodium azide thus reduces the hazards derived from the isolation and handling of the azides. A scanty literature is available for Huisgen 1,3-dipolar cycloaddition involving in situ generation of aryl azides.24 In these circumstances, initially a model reaction for Huisgen 1,3-dipolar cycloaddition of phenyldiazonium tetrafluoroborate, sodium azide and phenyl acetylene in water was carried out in presence of CuSO4·5H2O and catalytic amount of polymer supported ionic liquid (MR-IMZ-As) (Scheme 1). Pleasingly, corresponding 1,2,3-triazole was obtained in good yield within short time duration. By comparing the 1H NMR data, with the literature reports, the product was confirmed as 1,4-disubstituted 1,2,3-triazole instead of 1,5-disubstituted 1,2,3-triazole.25 Thus, the developed method act as a regioselective method.
For optimization of reaction conditions in terms of catalyst loading, model reaction was carried out with varied quantities of the catalyst. Best result was obtained using 25 mg (0.16 mmol) of catalyst in water at room temperature. We decided to employ these optimized conditions to probe the generality of the method. There was no difference in yield and reaction time when catalyst loading was increased to 30 mg (0.19 mmol). However, it took longer time for completion of the reaction when catalyst loading was reduced to 15 mg (0.09 mmol), 10 mg (0.064 mmol) or 5 mg (0.032 mmol). To check the effect of the catalyst and copper sulphate, a click reaction were performed without SILP and without copper sulphate, in both cases no product was obtained.
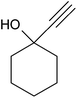
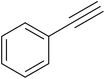
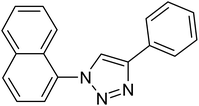
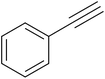
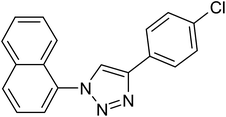
After optimization of the reaction conditions, to delineate this approach, particularly in regards to library construction, this methodology was evaluated by using a diversely substituted aryldiazonium tetrafluoroborate reacted with sodium azide and terminal alkyne to provide corresponding 1,4-disubstituted 1,2,3-triazoles under optimized reaction conditions. The results are summarized in Table 1. Electron donating substituents like methyl, methoxy (entries 1, 2, 14; Table 1), and electron withdrawing substituents such as chloro, nitro groups and cyano at para position of aryldiazonium tetrafluoroborate (entries 3, 4, 5, 13; Table 1) are found to be equally effective towards the nucleophilic substitution of in situ generated aryl azide, followed by 1,3-dipolar cycloaddition. It is noteworthy that when alicyclic acetylene, 4-methyl and 4-chloro phenylacetylene are selected as representative of terminated alkyne also undergo clean reaction to produce the corresponding 1,4-disubstituted 1,2,3-triazoles employing optimized reaction conditions (entries 6–12, 15, 16; Table 1).
| Entry | Diazonium salt | Alkyne | Product | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: aryldiazonium tetrafluoroborate (1 mmol), sodium azide (1 mmol), terminal alkyne (1 mmol), CuSO4·5H2O (10 mg, 0.04 mmol) and MR-IMZ-As (25 mg, 0.16 mmol) in water (5 mL).b Isolated yield. | |||||
| 1 |  |
 |
 |
2 | 94 |
| 2 | 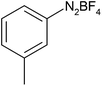 |
 |
 |
2 | 92 |
| 3 | 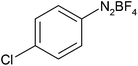 |
 |
 |
2 | 82 |
| 4 |  |
 |
 |
2 | 79 |
| 5 | 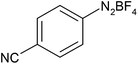 |
 |
 |
2 | 80 |
| 6 | 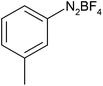 |
 |
 |
2 | 90 |
| 7 |  |
 |
 |
2 | 92 |
| 8 |  |
 |
 |
2 | 90 |
| 9 |  |
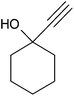 |
 |
2 | 96 |
| 10 |  |
 |
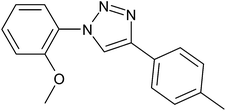 |
2 | 95 |
| 11 | 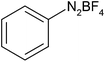 |
 |
 |
2 | 92 |
| 12 |  |
 |
 |
2 | 90 |
| 13 |  |
 |
 |
2 | 94 |
| 14 |  |
 |
 |
2 | 92 |
| 15 |  |
 |
 |
2 | 90 |
| 16 |  |
 |
 |
2 | 92 |
The reusability of the catalyst is an important aspect from economical point of view. So recovery and reusability study of the catalyst was performed for the reaction of 2-methoxyphenyldiazonium tetrafluoroborate, sodium azide and phenylacetylene. After the completion of the first reaction, catalyst was recovered by simple filtration and washed with ethanol and acetone, dried at 80 °C for 1 h. TEM-EDS analysis (Fig. 4) of the catalyst after first cycle indicated the presence of copper. This clearly indicates in situ incorporation of copper with polymer supported ascorbate functionalized task specific ionic liquid during the progress of reaction. From the XPS spectrum of catalyst, the values of binding energy at 932.3 and 954.7 eV corresponding to the Cu 2p3/2 and Cu 2p1/2 and peak centered at 531.3, 284.5 eV represent O 1s and C 1s, respectively. This data of binding energy is in agreement with the literature and revealed presence of CuO26a–c while existence of copper as Cu2+ is also confirmed by presence of the shake-up satellite peak26d,e with binding energy 940–950 eV (Fig. 5). Thus copper incorporated in the catalyst in the form of CuO. Hence reusability study was carried out in absence of CuSO4·5H2O. After 1st run copper content of the filtrate was determined using atomic absorption spectroscopy (AAS). No copper metal was detected in filtrate, which confirms the fact that polymer supported ascorbate functionalized task specific ionic liquid binds copper to minimize deterioration and metal leaching and facilitates efficient catalyst recycling. We observed that catalyst was reused at least for five times resulted 94, 94, 92, 90, 89 and 85% yield, respectively (Fig. 6). Metal leaching was also studied by TEM-EDS analysis of the catalyst before and after the 5th cycle. After 1st and 5th cycle the concentration of in situ incorporated Cu was found to be 3.33 wt% and 3.09 wt%, respectively.
Conclusion
We have described an efficient, regioselective and green procedure for the one-pot synthesis of 1,4-disubstituted 1,2,3-triazoles catalyzed by in situ incorporated copper with polymer supported ascorbate functionalized task specific ionic liquid in water at room temperature. This protocol provides advantages such as operational simplicity, general applicability, one-pot synthesis of triazoles from diazonium salts, high yields, involving room temperature and recyclability of the catalyst up to the fifth run without any appreciable loss of activity. High purity and yields of the product, excellent regioselectivity and circumventing the problems encountered with the isolation of organic azides, enable one to adhere to greener and cost effective approach for Huisgen cycloaddition.Experimental
General information
Alkynes (Aldrich/Alfa Aesar), sodium azide (Spectrochem, Mumbai) and copper sulphate pentahydrate (Spectrochem, Mumbai) were used as received. All reactions were carried out in air atmosphere in predried glassware. Infrared spectra were measured with an Agilent Cary (IR-630) spectrophotometer. 1H NMR and 13C NMR spectra were recorded on a Brucker AC spectrometer (300 MHz for 1H NMR and 75 MHz for 13C NMR), using CDCl3 as solvent and tetramethylsilane (TMS) as an internal standard. Chemical shifts (δ) are expressed in parts per million (ppm) and coupling constants are expressed in hertz (Hz). Mass spectra were recorded on a Shimadzu QP2010 GCMS. FESEM was performed using a HITACHI S-4800. TEM-EDS were determined using energy dispersive X-ray analyser EM912 coupled to transmission electron microscopy (TEM) JEOL-2100F transmission electron microscope. X-ray photoelectron (XPS) was recorded on VG Multilab ESCA 2000 system.Typical procedure for the preparation of polymer supported ascorbate functionalized ionic liquid catalyst (MR-IMZ-As)
Acknowledgements
We gratefully acknowledge the DST New Delhi for financial assistance under Fast Track Scheme [no. SB/FT/CS-154/2012].References
- (a) P. T. Anastas and J. C. Warner, Green Chemistry Theory and Practice, Oxford University Press, Oxford, 1998 Search PubMed; (b) A. S. Matlack, Introduction to Green Chemistry, Marcel Dekker, New York, 2001 Search PubMed; (c) M. Poliakoff, J. M. Fitzpatrick, T. R. Farren and P. T. Anastas, Science, 2002, 297, 807 CrossRef CAS PubMed; (d) M. Lancaster, Green Chemistry: An Introductory Text, RSC Publishing, Cambridge, 2002 Search PubMed; (e) R. A. Sheldon, I. W. C. E. Arends and U. Henefeld, Green Chemistry and Catalysis, Wiley-VCH, Weinheim, 2007 Search PubMed.
- (a) S. E. García-Garrido, J. Francos, V. Cadierno, J.-M. Basset and V. Polshettiwar, ChemSusChem, 2011, 4, 104 CrossRef PubMed; (b) A. Fihri, D. Cha, M. Bouhrara, N. Almana and V. Polshettiwar, ChemSusChem, 2012, 5, 85 CrossRef CAS PubMed; (c) N. Madhavan, C. W. Jones and M. Weck, Acc. Chem. Res., 2008, 41, 1153 CrossRef CAS PubMed; (d) A. Schätz, R. N. Grass, W. J. Stark and O. Reiser, Chem.–Eur. J., 2008, 14, 8262 CrossRef PubMed; (e) S. Bhadra, B. Sreedhar and B. C. Ranu, Adv. Synth. Catal., 2009, 351, 2369 CrossRef CAS.
- (a) J. D. Holbrey and K. R. Seddon, J. Chem. Soc., Dalton Trans., 1999, 2133 RSC; (b) T. Welton, Chem. Rev., 1999, 99, 2071 CrossRef CAS PubMed; (c) M. J. Earle, P. B. Mc Cormac and K. R. Seddon, Green Chem., 1999, 1, 23 RSC; (d) H. Olivier-Bourbigou, L. Magna and D. Morvan, Appl. Catal., A, 2010, 373, 1 CrossRef CAS PubMed; (e) J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508 CrossRef CAS PubMed.
- (a) M. T. Garcia, N. Gathergood and P. J. Scammells, Green Chem., 2005, 7, 9 RSC; (b) K. M. Docherty and C. F. Kulpa, Green Chem., 2005, 7, 185 RSC; (c) C. Pretti, C. Chiappe, D. Pieraccini, M. Gregori, F. Abramo, G. Monni and L. Intorre, Green Chem., 2006, 8, 238 RSC.
- (a) M. J. Earle, J. M. S. S. Esperanca, M. A. Gilea, J. N. C. Lopes, L. P. N. Rebelo, J. W. Magee, K. R. Seddon and J. A. Widegren, Nature, 2006, 72, 1391 Search PubMed; (b) A. W. Taylor, K. R. J. Lovelock, A. Deyko, P. Licence and R. G. Jones, Phys. Chem. Chem. Phys., 2010, 12, 1772 RSC.
- (a) A. Riisager, R. Fehrmann, M. Haumann and P. Wasserscheid, Top. Catal., 2006, 40, 91 CrossRef CAS PubMed; (b) C. Van Doorslaer, J. Wahlen, P. Mertens, K. Binnemans and D. D. Vos, Dalton Trans., 2010, 39, 8377 RSC; (c) C. P. Mehnert, Chem.–Eur. J., 2005, 11, 50 CrossRef PubMed.
- (a) H. Hagiwara, Y. Sugawara, K. Isobe, T. Hoshi and T. Suzuki, Org. Lett., 2004, 6, 2325 CrossRef CAS PubMed; (b) M. Gruttadauria, S. Riela, P. L. Meo, F. D'Anna and R. Noto, Tetrahedron Lett., 2004, 45, 6113 CrossRef CAS PubMed; (c) S. S. Shinde, B. S. Lee and D. Y. Chi, Tetrahedron Lett., 2008, 49, 4245 CrossRef CAS PubMed; (d) J. Joni, M. Haumann and P. Wasserscheid, Adv. Synth. Catal., 2009, 351, 423 CrossRef CAS; (e) H. Hagiwara, Synlett, 2012, 23, 837 CrossRef CAS PubMed.
- (a) M. Whiting, J. Muldoon, Y.-C. Lin, S. M. Silverman, W. Lindstrom, A. J. Olson, H. C. Kolb, M. G. Finn, K. B. Sharpless, J. H. Elder and V. V. Fokin, Angew. Chem., Int. Ed., 2006, 45, 1435 CrossRef CAS PubMed; (b) H. C. Kolb and K. B. Sharpless, Drug Discovery Today, 2003, 8, 1128 CrossRef CAS; (c) M. J. Giffin, H. Heaslet, A. Brik, Y. C. Lin, G. Cauvi, C.-H. Wong, D. E. McRee, J. H. Elder, C. D. Stout and B. E. Torbett, J. Med. Chem., 2008, 51, 6263 CrossRef CAS PubMed; (d) W.-Q. Fann and A. R. Katritzky, in Comprehensive Heterocyclic Chemistry II, ed. A. R. Katritzky, C. W. Rees and E. F. V. Scriven, Elsevier Science, Oxford, 1996, vol. 4, p. 1 Search PubMed.
- D. R. Buckle, C. J. M. Rockell, H. Smith and B. A. Spicer, J. Med. Chem., 1986, 29, 2262 CrossRef CAS.
- (a) F. Pagliai, T. Pirali, E. D. Grosso, R. D. Brisco, G. C. Tron, G. Sorba and A. A. Genazzani, J. Med. Chem., 2006, 49, 467 CrossRef CAS PubMed; (b) S. A. Bakunov, S. M. Bakunova, T. Wenzler, M. Ghebru, K. A. Werbovetz, R. Brun and R. R. Tidwell, J. Med. Chem., 2010, 53, 254 CrossRef CAS PubMed; (c) A. H. Banday, S. A. Shameem, B. D. Gupta and H. M. Sampath Kumar, Steroids, 2010, 75, 801 CrossRef CAS PubMed.
- R. Alvarez, S. Velazquez, A. San-Felix, S. Aquaro, E. De Clercq, C.-F. Perno, A. Karlsson, J. Balzarini and M. J. Camarasa, J. Med. Chem., 1994, 37, 4194 CrossRef.
- L. L. Brockunier, E. R. Parmee, H. O. Ok, M. R. Candelore, M. A. Cascieri, L. F. Colwell, L. Deng, W. P. Feeney, M. J. Forrest, G. J. Hom, D. E. MacIntyre, L. Tota, M. J. Wyvratt, M. H. Fisher and A. E. Weber, Bioorg. Med. Chem. Lett., 2000, 10, 2111 CrossRef CAS.
- (a) E. Haldón, E. Álvarez, M. C. Nicasio and P. J. Pérez, Chem. Commun., 2014, 50, 8978 RSC; (b) X. Liu, J. Li and B. Chen, New J. Chem., 2013, 37, 965 RSC; (c) J. D. Patil and D. M. Pore, RSC Adv., 2014, 4, 14314 RSC; (d) D. M. Pore, P. G. Hegade, M. M. Mane and J. D. Patil, RSC Adv., 2013, 3, 25723 RSC; (e) S. Ueda and H. Nagasawa, J. Am. Chem. Soc., 2009, 131, 15080 CrossRef CAS PubMed; (f) D. V. Batchelor, D. M. Beal, T. B. Brown, D. Ellis, D. W. Gordon, P. S. Johnson, H. J. Mason, M. J. Ralph, T. J. Underwood and S. Wheeler, Synlett, 2008, 2421 CAS; (g) P. Yin, W.-B. Ma, Y. Chen, W.-C. Huang, Y. Deng and L. He, Org. Lett., 2009, 11, 5482 CrossRef CAS PubMed.
- (a) V. Sai Sudhir, R. B. Nasir Baig and S. Chandrasekaran, Eur. J. Org. Chem., 2008, 2423 CrossRef CAS; (b) V. Sai Sudhir, N. Y. Phani Kumar, R. B. Nasir Baig and S. Chandrasekaran, J. Org. Chem., 2009, 74, 7588 CrossRef CAS PubMed; (c) F. Amblard, J. H. Cho and R. F. Schinazi, Chem. Rev., 2009, 109, 4207 CrossRef CAS PubMed; (d) S. Dedola, S. A. Nepogodiev and R. A. Field, Org. Biomol. Chem., 2007, 5, 1006 RSC; (e) E. M. Sletten and C. R. Bertozzi, Acc. Chem. Res., 2011, 44, 666 CrossRef CAS PubMed; (f) D. Kumar and V. B. Reddy, Synthesis, 2010, 1687 CrossRef CAS PubMed; (g) R. B. N. Baig and R. S. Varma, Chem. Commun., 2012, 48, 5853 RSC; (h) V. Ganesh, V. S. Sudhir, T. Kundu and S. Chandrasekaran, Chem.–Asian J., 2011, 6, 2670 CrossRef CAS PubMed; (i) D. Kumar, G. Patel and V. B. Reddy, Synlett, 2009, 399 CrossRef CAS PubMed; (j) D. Kumar, V. B. Reddy, A. Kumar, D. Mandal, R. Tiwari and K. Parang, Bioorg. Med. Chem. Lett., 2011, 21, 449 CrossRef CAS PubMed.
- (a) R. Huisgen, Proc. Chem. Soc., London, 1961, 357 Search PubMed; (b) 1,3-Dipolar Cycloaddition Chemistry, ed. R. Huisgen and A. Pawda, Wiley, New York, 1984, p. 1 Search PubMed; (c) R. Huisgen, Pure Appl. Chem., 1989, 61, 613 CrossRef CAS; (d) V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef CAS; (e) C. Chiappe, B. Mennucci, C. S. Pomelli, A. Sanzone and A. Marra, Phys. Chem. Chem. Phys., 2010, 12, 1958 RSC.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef CAS.
- P. Appukkuttan, W. Dehaen, E. Van der Eycken and V. V. Fokin, Org. Lett., 2004, 6, 4223 CrossRef CAS PubMed.
- (a) N. Mukherjee, S. Ahmmed, S. Bhadra and B. C. Ranu, Green Chem., 2013, 15, 389 RSC; (b) R. B. N. Baig and R. S. Varma, Green Chem., 2012, 14, 625 RSC; (c) B. S. P. A. Kumar, K. H. V. Reddy, B. Madhav, K. Ramesh and Y. V. D. Nageswar, Tetrahedron Lett., 2012, 53, 4595 CrossRef PubMed; (d) R. Hudson, C. J. Li and A. Moores, Green Chem., 2012, 14, 622 RSC; (e) J.-A. Shin, Y.-G. Lim and K.-H. Lee, J. Org. Chem., 2012, 77, 4117 CrossRef CAS PubMed; (f) J. Andersen, S. Bolvig and X. Liang, Synlett, 2005, 2941 CAS; (g) J. Yan and L. Wang, Synthesis, 2010, 447 CAS; (h) Y.-B. Zhao, Z.-Y. Yan and Y.-M. Liang, Tetrahedron Lett., 2006, 47, 1545 CrossRef CAS PubMed; (i) B. J. Borah, D. Dutta, P. P. Saikia, N. C. Barua and D. K. Dutta, Green Chem., 2011, 13, 3453 RSC.
- (a) K. Kamata, Y. Nakagawa, K. Yamaguchi and N. Mizuno, J. Am. Chem. Soc., 2008, 130, 15304 CrossRef CAS PubMed; (b) A. Kolarovič, M. Schnürch and M. D. Mihovilovic, J. Org. Chem., 2011, 76, 2613 CrossRef PubMed; (c) S. Karlsson and H. E. Hogberg, Org. Prep. Proced. Int., 2001, 33, 103 CrossRef CAS.
- (a) C. Girard, E. Onen, M. Aufort, S. Beauviere, E. Samson and J. Herscovici, Org. Lett., 2006, 8, 1689 CrossRef CAS PubMed; (b) S. Chassaing, M. Kumarraja, A. S. S. Sido, P. Pale and J. Sommer, Org. Lett., 2007, 9, 883 CrossRef CAS PubMed; (c) T. Miao and L. Wang, Synthesis, 2008, 363 CAS; (d) S. Chassaing, A. S. Sido, A. Alix, M. Kumarraja, P. Pale and J. Sommer, Chem.–Eur. J., 2008, 14, 6713 CrossRef CAS PubMed; (e) B. H. Lipshutz and B. R. Taft, Angew. Chem., Int. Ed., 2006, 45, 8235 CrossRef CAS PubMed; (f) B. Movassagh and N. Rezaei, Tetrahedron, 2014, 70, 8885 CrossRef CAS PubMed; (g) I. Jlalia, F. Gallier, N. Brodie-Linder, J. Uziel, J. Augé and N. Lubin-Germain, J. Mol. Catal. A: Chem., 2014, 393, 56 CrossRef CAS PubMed.
- (a) H. Sharghi, R. Khalifeh and M. M. Doroodmand, Adv. Synth. Catal., 2009, 351, 207 CrossRef CAS; (b) F. Alonso, Y. Moglie, G. Radivoy and M. Yus, Adv. Synth. Catal., 2010, 352, 3208 CrossRef CAS; (c) D. Raut, K. Wankhede, V. Vaidya, S. Bhilare, N. Darwatkar, A. Deorukhkar, G. Trivedi and M. Salunkhe, Catal. Commun., 2009, 10, 1240 CrossRef CAS PubMed.
- S. Brase, C. Gil, K. Knepper and V. Zimmermann, Angew. Chem., Int. Ed., 2005, 44, 5188 CrossRef CAS PubMed.
- (a) J. T. Fletcher and J. E. Reilly, Tetrahedron Lett., 2011, 52, 5512 CrossRef CAS PubMed; (b) K. Barral, A. D. Moorhouse and J. E. Moses, Org. Lett., 2007, 9, 1809 CrossRef CAS PubMed; (c) K. Odlo, E. A. Hoydahl and T. V. Hansen, Tetrahedron Lett., 2007, 48, 2097 CrossRef CAS PubMed; (d) A. K. Feldman, B. Colasson and V. V. Fokin, Org. Lett., 2004, 6, 3897 CrossRef CAS PubMed; (e) N. M. Smith, M. J. Greaves, R. Jewell, M. V. D. Perry, M. J. Stocks and J. P. Stonehouse, Synthesis, 2009, 1391 CAS.
- (a) S. Roy, T. Chatterjee and S. M. Islam, Green Chem., 2013, 15, 2532 RSC; (b) F. Alonso, Y. Moglie, G. Radivoy and M. Yus, Org. Biomol. Chem., 2011, 9, 6385 RSC; (c) A. Zarei, Tetrahedron Lett., 2012, 53, 5176 CrossRef CAS PubMed.
- L. Zhang, X. Chen, P. Xue, H. H. Y. Sun, I. D. Williams, K. B. Sharpless, V. V. Fokin and G. Jia, J. Am. Chem. Soc., 2005, 127, 15998 CrossRef CAS PubMed.
- (a) J. Morales, J. P. Espinos, A. Caballero, A. R. Gonzalez-Elipe and J. A. Mejias, J. Phys. Chem. B, 2005, 109, 7758 CrossRef CAS PubMed; (b) J. P. Espinos, J. Morales, A. Barranco, A. Caballero, J. P. Holgado and A. R. Gonzalez-Elipe, J. Phys. Chem. B, 2002, 106, 6921 CrossRef CAS; (c) N. Toshima and Y. Wang, Langmuir, 1994, 10, 4574 CrossRef CAS; (d) S. Roy, T. Chatterjee, B. Banerjee, N. Salam, A. Bhaumik and Sk M. Islam, RSC Adv., 2014, 4, 46075 RSC; (e) G. Zhang, J. Long, X. Wang, W. Dai, Z. Li, L. Wu and X. Su, New J. Chem., 2009, 33, 2044 RSC; (f) Y. Wan, J. Ma, Z. Wang, W. Zhou and S. Kaliaguine, Appl. Catal., B, 2005, 59, 235 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra16481d |
| This journal is © The Royal Society of Chemistry 2015 |