Transition metal induced switch of fluorescence and absorption response of a Zn(ii)porphyrin–DNA conjugate to cysteine derivatives†
Abstract
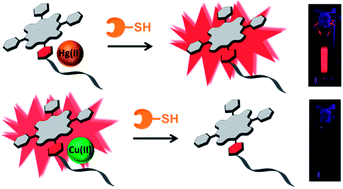
We report a supramolecular zinc(II)tetraarylporphyrin–oligothymidine/metal ion system (ZnPorT8/M2+) for dual optical sensing of cysteine (Cys) and glutathione (GSH) with a tunable spectroscopic outcome. Transition metal ions (M2+ = Hg2+ or Cu2+) allow the switching of the emission and absorption response of the ZnPorT8/M2+ complex. The ZnPorT8/Hg2+ complex exhibits turn-on fluorescence sensing (fluorescence enhancement), whilst the ZnPorT8/Cu2+ system exhibits turn-off sensing (emission quenching) of Cys and GSH. The ZnPorT8/Hg2+ and ZnPorT8/Cu2+ complexes have an excellent limit of detection (LOD(em)) for Cys (5.95 nM and 11.99 nM, respectively) and GSH (3.34 nM and 13.50 nM, respectively).

 Please wait while we load your content...
Please wait while we load your content...