Glycosylation of polyphosphazenes by thiol-yne click chemistry for lectin recognition†
Abstract
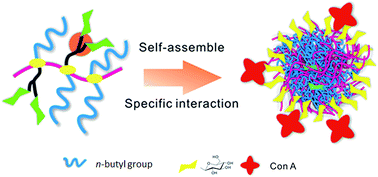
Strong carbohydrate–lectin binding interactions in biological systems can be mimicked through the synthesis of glucose containing macromolecules, particularly glycosylated polymers. Herein, an amphiphilic glycosylated polyphosphazene was synthesized by mixed substitution of poly(dichlorophosphazene) with n-butylamine and propargylamine, and a subsequent “thiol-yne” click reaction between poly[(propargylamine/n-butylamine)phosphazene] (PPBP) and 2,3,4,6-tetra-O-acetyl-1-thio-β-D-glucopyranose. Polyphosphazenes with different glycosyl densities, P-37% and P-58%, were obtained and investigated. It was found that these amphiphilic glycopolymers could self-assemble to form global micelles in aqueous solution. An increase in size occurred when the polyphosphazenes interacted with concanavalin A (Con-A). Such interaction was further confirmed by variations in absorbance demonstrated by ultraviolet-visible spectroscopy. Disaggregation of the polyphosphazene–Con-A complex was observed in the presence of free glucose, which was possibly due to variable ratios of glycosyl-to-alkyl moieties in the glycosylated PPBP. The glucosylated polyphosphazene bound specifically to Con-A but not to peanut agglutinin, as determined by fluorescence microscopy.

 Please wait while we load your content...
Please wait while we load your content...