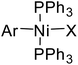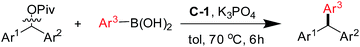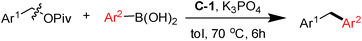Ni(II) source as a pre-catalyst for the cross-coupling of benzylic pivalates with arylboronic acids: facile access to tri- and diarylmethanes†
Qiang Chenab,
Xin-Heng Fan*a,
Li-Peng Zhangab and
Lian-Ming Yang*a
aBeijing National Laboratory for Molecular Sciences (BNLMS), Key Laboratory of Green Printing, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China. E-mail: yanglm@iccas.ac.cn; xinxin9968@iccas.ac.cn; Fax: +86-10-62559373
bUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 23rd January 2015
Abstract
A simple and easily-used NiII complex, Ni(PPh3)2(1-naphthyl)Cl, was employed as a pre-catalyst in the Suzuki–Miyaura cross-coupling of benzylic pivalates with arylboronic acids, affording various tri- or diarylmethanes in good yields under mild conditions. This new protocol provides a cheap, convenient and practical alternative to synthesizing multiaryl methanes.
The study of methodologies for the construction of multi-aryl methanes has been attracting strong interest from synthetic chemists because these target molecules are valuable frameworks in medicinal and materials chemistry, as well as organic synthesis.1 Among the synthetic strategies developed, the Suzuki–Miyaura reaction of benzylic electrophiles has emerged as a highly preferred method.2–10 In this regard, successful examples included cross-couplings of organoboron reagents with benzylic halides,2 carbonates,3 acetates,4 phosphates5 and sulfones6 (Pd-catalyzed processes), as well as with benzylic ammonium salts,7 carbamates,8 pivalates9 and ethers10 (Ni0-catalyzed processes). Despite the impressive advances, improvement on the catalyst systems of those protocols remains urgently needed, since it was recognized that palladium belongs to precious metals, and the Ni0 species is difficult to experimentally handle due to its high air-sensitivity and toxicity.
trans-Haloarylbis(triphenylphosphane)nickel(II) (Fig. 1) are a special type of nickel(II) compounds, many of which can be conveniently prepared from cheap, commercially available starting materials, and display better stability in air and moisture.11 We previously confirmed that NiII–(σ-aryl) complexes are highly applicable catalyst precursors in Suzuki–Miyaura-type cross-couplings.12 Therefore, we envisioned that the use of such nickel(II) pre-catalysts would be feasible in cross-couplings of benzylic pivalates with arylboronic acids. Herein, we wish to disclose our new findings.
We first attempted the cross-coupling of a diarylmethyl pivalate 1 (belonging to a type of secondary benzylic pivalates) with p-anisylboronic acid under the NiII–(σ-aryl) complex catalysis. Ni(PPh3)2(1-naphthyl)Cl (C-1) was preferably selected as pre-catalyst, as previously in our laboratory.12 After some experimentation, the reaction was found to proceeded smoothly with a high isolated yield of 86% (entry 1), and over-loading ligands was virtually unfavourable for the reaction (entries 2–5). As expected, other types of nickel(II) sources, such as NiCl2·6H2O, Ni(acac)2 and Ni(Ph3P)2Cl2, were ineffective for the desired C–C coupling (entries 6–8). This may be because common NiII precursors are unable to produce the catalytically active Ni0 species in the reaction system.12a The type of bases is also important (entry 1 vs. entries 9 and 10). Toluene appeared to be the solvent of choice for the reaction and far superior to ethereal solvents such as dioxane (entry 11) and THF (entry 12). In addition, reducing the catalyst loading (entry 13) or lowering the reaction temperature (entry 14) would cause a dramatic decrease in yields. Finally, the role of the NiII complex in the reaction was demonstrated clearly in a control experiment (entry 15) (Table 1).
| Entry | [Ni(II)] (mol%) | Ligand (mol%) | Base | Solvent | Temp. (°C) | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Conditions: the pivalate 1 (1.0 mmol), p-anisylboronic acid (1.5 mmol), base (2.5 mmol), 5.0 mL of solvent, 6 h, N2.b Isolated yields.c C-1: Ni(PPh3)2(1-naphthyl)Cl.d C-2: NiCl2·6H2O.e C-3: Ni(acac)2.f C-4: NiCl2(PPh3)2. | ||||||
| 1 | C-1c (5) | None | K3PO4 | Toluene | 70 | 86 |
| 2 | C-1 (5) | PPh3 (10) | K3PO4 | Toluene | 70 | 41 |
| 3 | C-1 (5) | PCy3 (10) | K3PO4 | Toluene | 70 | 46 |
| 4 | C-1 (5) | DPPP (5) | K3PO4 | Toluene | 70 | 19 |
| 5 | C-1 (5) | DPPF (5) | K3PO4 | Toluene | 70 | 27 |
| 6 | C-2d (5) | None | K3PO4 | Toluene | 70 | 0 |
| 7 | C-3e (5) | None | K3PO4 | Toluene | 70 | 0 |
| 8 | C-4f (5) | None | K3PO4 | Toluene | 70 | 0 |
| 9 | C-1 (5) | None | K2CO3 | Toluene | 70 | 12 |
| 10 | C-1 (5) | None | CsF | Toluene | 70 | 7 |
| 11 | C-1 (5) | None | K3PO4 | Dioxane | 70 | 9 |
| 12 | C-1 (5) | None | K3PO4 | THF | 70 | Trace |
| 13 | C-1 (2.5) | None | K3PO4 | Toluene | 70 | 39 |
| 14 | C-1 (5) | None | K3PO4 | Toluene | 50 | 61 |
| 15 | C-1 (0) | None | K3PO4 | Toluene | 70 | 0 |
Next, the scope and limitations of this NiII-catalyzed benzyl–aryl coupling reactions were investigated. Under the optimized conditions we carried out the reaction of diarylmethyl pivalates (Table 2) and of primary benzylic pivalates (Table 3), respectively, with arylboronic acids.
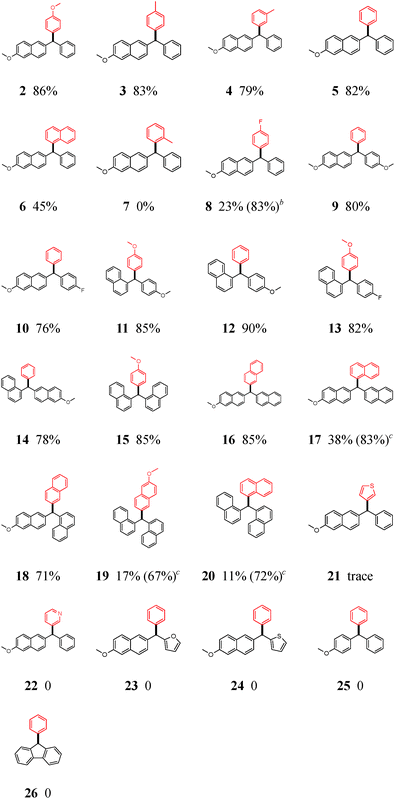
Regarding the boronic acid component, both electron-rich (2–4, 11, 13, and 15) and -neutral (5, 9, 10, 12, 14, 16, and 18) arylboronic acids showed excellent reactivity, providing desired products in high yields. Electron-deficient p-fluorophenylboronic acid (8) performed poorly under the standard conditions, but the outcome was improved by increasing its amounts (8). The reason may be that electron-deficient boronic acids are less nucleophilic and undergo a slower transmetallation as compared to electron-rich and -neutral ones. This coupling reaction is also very sensitive to the steric effects of arylboronic acids: 1-naphthyl boronic acid gave a lower yield (6, 17, and 20); the ortho-substituted substrate completely retarded the reaction (7). With respect to diarylmethyl pivalates, at least one aryl should belong to fused aromatic rings such as naphthyl group,13 or else the reaction does not occur (comparing 25 and 26 with other cases in Table 2). For the second aryl group, the limitation is relatively less: whether electron-neutral (2–5), -rich (9, 11, and 12), or -poor (10 and 13) ones can offer good yields of the desired products, with the exception of heteroaryls (23 and 24). Dinaphthyl-substituted substrates appeared more favorable for the reaction (14–20), since even sterically congested coupling reactions (17, 19, and 20) proceeded smoothly under the slightly modified conditions.
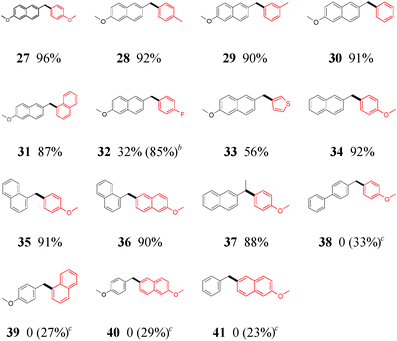
To our delight, the optimized conditions can be extended with no difficulty to the Suzuki–Miyaura cross-coupling of primary benzylic pivalates for diarylmethane synthesis (Table 3). A wider array of arylboronic acids, including electron-rich (27–29, and 34–37), -neutral (30 and 31), -deficient (32) and even heteroaryl (33) boronic acid, can be utilized in the reaction with good to excellent yields. Similarly, only benzylic pivalate containing the naphthyl substructure gave a satisfactory outcome (27–37).13 Benzylic pivalates with no naphthyl group were not coupled under our standard conditions, but a sluggish conversion was achieved upon the use of DPPF as an additional ligand in the NiII catalyst system (38–41). Notably, a high yield of diarylethane 37 was obtained without β-hydride elimination.
The mechanism of the reaction is believed to be almost the same as that of the well-established nickel-catalyzed Suzuki–Miyaura cross-coupling reaction: that is a typical catalytic cycle of the Ni0–NiII shuttle involving sequential oxidative addition of the Ni0 (in situ generated from the Ni(PPh3)2(1-naphthyl)Cl precursor12) into benzylic C–O bond, transmetallation, and reductive elimination.
In summary, we have demonstrated a facile route to tri- and diarylmethanes by a nickel-catalyzed Suzuki–Miyaura cross-coupling reaction of benzylic pivalates. This new protocol is characteristic of no using nickel(0) sources and special ligands in Ni-based catalyst systems. Further work to expand the scope of substrates and elucidate the mechanistic details is currently underway in our lab.
Acknowledgements
We thank National Natural Science Foundation of China (Project nos 20872142 and 21102150) for financial support of this work.Notes and references
- (a) A very recent comprehensive review: S. Mondal and G. Panda, RSC Adv., 2014, 4, 28317 RSC; (b) V. Nair, S. Thomas, S. C. Mathew and K. G. Abhilash, Tetrahedron, 2006, 62, 6731 CrossRef CAS PubMed; (c) M. S. Shchepinov and V. A. Korshun, Chem. Soc. Rev., 2003, 32, 170 RSC; (d) D. F. Duxbury, Chem. Rev., 1993, 93, 381 CrossRef CAS.
- (a) S. Chowdhury and P. E. Georghiou, Tetrahedron Lett., 1999, 40, 7599 CrossRef CAS; (b) L. Botella and C. Nájera, Angew. Chem., Int. Ed., 2002, 41, 179 CrossRef CAS; (c) S. Langle, M. Abarbri and A. Duchêne, Tetrahedron Lett., 2003, 44, 9255 CrossRef CAS PubMed; (d) B. P. Bandgar, S. V. Bettigeri and J. Phopase, Tetrahedron Lett., 2004, 45, 6959 CrossRef CAS PubMed; (e) S. M. Nobre and A. L. Monteiro, Tetrahedron Lett., 2004, 45, 8225 CrossRef CAS PubMed; (f) G. A. Molander and M. D. Elia, J. Org. Chem., 2004, 69, 3447 CrossRef PubMed; (g) N. Henry, C. Enguehard-Gueiffier, I. Thery and A. Gueiffier, Eur. J. Org. Chem., 2008, 4824 CrossRef CAS; (h) B. Inés, I. Moreno, R. SanMartin and E. Domínguez, J. Org. Chem., 2008, 73, 8448 CrossRef PubMed; (i) D. Srimani and A. Sarkar, Tetrahedron Lett., 2008, 49, 6304 CrossRef CAS PubMed; (j) A. Yu, X. Li, D. Peng, Y. Wu and J. Chang, Appl. Organomet. Chem., 2012, 26, 301 CrossRef CAS.
- (a) R. Kuwano and M. Yokogi, Org. Lett., 2005, 7, 945 CrossRef CAS PubMed; (b) J.-Y. Yu and R. Kuwano, Org. Lett., 2008, 10, 973 CrossRef CAS PubMed.
- R. Kuwano and M. Yokogi, Comm. Commun., 2005, 5899 CAS.
- M. McLaughlin, Org. Lett., 2005, 7, 4875 CrossRef CAS PubMed.
- M. Nambo and C. M. Crudden, Angew. Chem., Int. Ed., 2014, 53, 742 CrossRef CAS PubMed.
- P. Maity, D. M. Shacklady-McAtee, G. P. A. Yap, E. R. Sirianni and M. P. Watson, J. Am. Chem. Soc., 2013, 135, 280 CrossRef CAS PubMed.
- M. R. Harris, L. E. Hanna, M. A. Greene, C. E. Moore and E. R. Jarvo, J. Am. Chem. Soc., 2013, 135, 3303 CrossRef CAS PubMed.
- Q. Zhou, H. D. Srinivas, S. Dasgupta and M. P. Watson, J. Am. Chem. Soc., 2013, 135, 3307 CrossRef CAS PubMed.
- M. Tobisu, A. Yasutome, H. Kinuta, K. Nakamura and N. Chatani, Org. Lett., 2014, 16, 5572 CrossRef CAS PubMed.
- For the preparation of trans-haloarylbis(triphenylphosphane)nickel(II), see: (a) J. van Soolingen, H. D. Verkruijsse, M. A. Keegstra and L. Brandsma, Synth. Commun., 1990, 20, 3153 CrossRef CAS; (b) L. Brandsma, S. F. Vasilevsky and H. D. Verkruijsse, in Application of Transition Metal Catalysts in Organic Synthesis, Springer, New York, 1998, pp. 3–4 Search PubMed.
- (a) C. Chen and L.-M. Yang, Tetrahedron Lett., 2007, 48, 2427 CrossRef CAS PubMed; (b) X.-H. Fan and L.-M. Yang, Eur. J. Org. Chem., 2010, 2457 CrossRef CAS; (c) X.-H. Fan and L.-M. Yang, Eur. J. Org. Chem., 2011, 1467 CrossRef CAS; (d) Q. Chen, X.-H. Fan, L.-P. Zhang and L.-M. Yang, RSC Adv., 2014, 4, 53885 RSC.
- Generally, benzylic electrophiles without a fused aromatic show less reactive in Ni-catalyzed cross-couplings as well as in some Pd-catalyzed cross-couplings: (a) M. A. Greene, I. M. Yonova, F. J. Williams and E. R. Jarvo, Org. Lett., 2012, 14, 4293 CrossRef CAS PubMed; (b) S. Tabuchi, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2014, 79, 5401 CrossRef CAS PubMed and references cited therein; (c) Also see: ref. 8–10.
Footnote |
| † Electronic supplementary information (ESI) available: General experimental procedures, characterization details, 1H and 13C NMR spectra of products. See DOI: 10.1039/c4ra16452k |
| This journal is © The Royal Society of Chemistry 2015 |