Simultaneous colorimetric detection of four drugs in their pharmaceutical formulations using unmodified gold nanoparticles as a probe†
Abstract
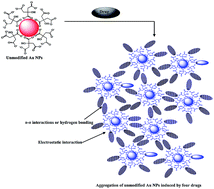
We have developed a simple UV-visible spectrometric method for parallel detection of four drugs (venlafaxine, imipramine, amlodipine and alfuzosin) by using unmodified gold nanoparticles (Au NPs) as a colorimetric probe. The citrate was self-assembled onto the Au NPs to form a probe that undergoes a color change from red to blue by the addition of the four drugs. It is presumed that the color change is a result of the aggregation of the Au NPs induced by the four drugs, resulting a red-shift in their absorption spectra from 521 to 653, 695, 688 and 636 nm for venlafaxine, imipramine, amlodipine and alfuzosin, respectively. The method was validated in the concentration range of 0.001–100 μM, where it demonstrated good linearity (R2 = 0.997, 0.997, 0.995 and 0.998) with limits of detection in the range of 0.9–9.3 nM. The aggregation of the Au NPs induced by the four drugs was confirmed by dynamic light scattering (DLS) and transmission electron microscopy (TEM). The applicability of the method was demonstrated by determining the drugs contents in pharmaceutical and biological samples (urine and plasma).

 Please wait while we load your content...
Please wait while we load your content...