The impact of the HPMA polymer structure on the targeting performance of the conjugated hydrophobic ligand†
Qingqing Yang,
Lian Li,
Xi Zhu,
Wei Sun,
Zhou Zhou and
Yuan Huang*
Key Laboratory of Drug Targeting and Drug Delivery System, Ministry of Education, West China School of Pharmacy, Sichuan University, No. 17, Block 3, Southern Renmin Road, Chengdu 610041, P.R. China. E-mail: huangyuan0@163.com; Fax: +86-28-85501617; Tel: +86-28-85501617
First published on 23rd January 2015
Abstract
Thanks to the enhanced cancer cell affinity, modification of polymeric drug carriers with an active targeting ligand has been one of the most effective strategies to achieve better therapeutic efficacy. However, hydrophobic ligands (e.g. folate), when conjugated to hydrophilic carriers, encounter the problem of reduced targeting effectiveness due to the fact that the ligand may be buried inside the carrier. In the present study, we studied the impact of the hydrophilic polymer structure on the targeting efficiency of the conjugated folate (FA). After modification with folate, the linear N-(2-hydroxypropyl)-methacrylamide (HPMA) polymer (P-FA, 27.3 kDa) exhibited a neutral surface charge (1.5 mV), while the graft HPMA polymer (GP-FA, 117.4 kDa) possessed a mild negative charge (−7.2 mV), which suggests different exposure levels of negatively charged folate on the surface of P-FA and GP-FA. Notably, on folate receptor positive MCF-7 cells, P-FA exhibited comparable cell uptake with the unmodified linear polymer, whereas GP-FA resulted in 2-fold higher cell internalization over the untargeted graft polymer. Meanwhile, the involvement of folate receptor mediated endocytosis and augmented folate binding affinity of GP-FA were observed in comparison with P-FA. Moreover, GP-FA exhibited significantly enhanced tumor accumulation relative to GP, while marginally improved tumor accumulation was observed for P-FA compared with P. In summary, the structure of the hydrophilic polymer plays a vital role in the targeting efficiency of the conjugated hydrophobic ligand.
Introduction
Over the past few decades, the application of macromolecular drug carriers, especially hydrophilic polymers, for the targeted delivery of therapeutic agents in anticancer treatment has achieved remarkable progress.1–4 Chemotherapeutic agents that conjugated onto hydrophilic polymers exhibit significantly improved therapeutic efficacy and reduced toxicity as a result of ameliorated pharmacokinetic behavior and increased passive tumor accumulation via the enhanced permeability and retention (EPR) effect.3,5 More recently, an active targeting strategy has been proposed and intensively studied to further increase the therapeutic efficacy of the polymer–drug conjugates. Introduction of targeting ligands, which specifically bind to receptors that overexpressed on tumor cells, has been demonstrated to effectively improve the tumor cell internalization of the conjugates.6,7However, the targeting effect of ligand conjugated on polymeric drug carriers is largely determined by their steric accessibility to receptor, which can be influenced by a lot of factors, such as the physiochemical property of the ligand.8,9 In particular, hydrophobic ligand, when conjugated to hydrophilic carriers, encountered the problem of limited exposure, as the ligand was highly likely to be embedded inside the macromolecular carrier.10–12 For example, the folate modified PEG (polyethylene glycol)–epirubicin conjugates showed even less cytotoxicity than the unmodified ones on folate receptor positive cells,11 indicating the hydrophobicity of folates affect their exposure on the surface of polymer and accessibility to receptor. Although increasing the folate content to high level may bring about saturated folate concentration in the hydrophobic core of hydrophilic polymer chain and can increase the amount of folate presented on the surface, this may also even be detrimental to the passive tumor accumulation of the carrier in vivo as the reduced water solubility and accelerated blood clearance.13,14
Another strategy to further increase the in vivo efficacy of polymer–drug conjugates is the synthesis of biodegradable high molecular weight (MW) polymers, such as branched,15 graft,16 and star polymers.17 Since these high MW polymers possess a size larger than the renal threshold, higher tumor accumulation can be achieved via EPR effect as a result of the further reduced renal clearance rate and improved long-circulating property. Besides, compared with linear polymer, these high MW polymers with more complicated hydrophilic structure may alter the steric accessibility of the conjugated ligands to receptor on the cell membrane. Nevertheless, the role of polymer architecture in targeting performance of conjugated ligand has received little attention.
Therefore, with the aim to gain some insight of the influence of hydrophilic polymer structure on the targeting efficiency of the conjugated ligand, we herein for the first time investigated the in vitro and in vivo behaviors of low MW linear polymer and high MW graft polymer, which were both modified with folate, a widely used hydrophobic targeting agent.18,19 Water-soluble N-(2-hydroxypropyl)-methacrylamide (HPMA) polymers were used as the model polymer, which have emerged as one of the most promising drug carriers due to their excellent water solubility and easy modification property.20–23 The cellular internalization and endocytic mechanism on folate receptor overexpressed MCF-7 human breast cancer cell were studied. For in vivo evaluation, the pharmacokinetics and biodistribution following systemic injection were investigated on mice bearing MCF-7 tumor xenografts.
Experimental section
Materials
Wortmannin was purchased from Selleck Chemicals (Shanghai, China). The primary antibody MAb MOv18 and secondary antibody FITC-conjugated goat anti-rabbit IgG (H+L) were purchased from ABclonal. Cy5.5-NHS ester was bought from Lumiprobe (Hallandale beach, FL, USA). 2-Iminothiolane hydrochloride was purchased from Toronto Research Chemicals (Toronto, Ontario, Canada). The following reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA): folic acid (FA), 2,4,6-trinitrobenzene-1-sulfonic acid (TNBSA), 3-(4,5-dimethyl-2-tetrazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), fluorescein isothiocyanate (FITC), reduced glutathione (GSH), dithiothreitol (DTT), 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB), 4′,6-diamidino-2-phenylindole (DAPI), papain and rottlerin. All the other reagents and solvents were purchased from Aladdin Reagent Co., Ltd. (Shanghai, China) and used as received.Synthesis and characterization of monomers
N-(2-Hydroxypropyl)-methacrylamide (HPMA),24 N-methacryloyl-glycylglycyl-p-nitrophenyl ester (MA-GG-ONp),25 N-methacryloyl-glycylphenylalanylleucylglycine (MA-GFLG-OH),24 N-methacryloyl-aminopropyl-fluorescein-5-isothiocyanate(MA-AP-FITC),26 N-(tert-butoxycarbonyl)-N′-(6-methacrylamido-hexanoyl)-hydrazine (Ma-ah-NHNH-Boc)27 and 3-(N-methacryloylglycyl)-DL-phenylalanylleucylglycyl-thiazolidine-2-thione (Ma-GFLG-TT)28 were synthesized and characterized according to previously established protocols.Synthesis and characterization of 3,3′-[4,4′-azobis(4-cyano-4-methyl-1-oxo-butane-4,1-diyl)]bis(thiazolidine-2-thione) (ABIK-TT), N-methacryloyl-glycylglycyl-propargyl (MA-GG-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH), 2-(2-pyridyldithio)-ethylamine hydrochloride (PDEA), azido-modified folate are described in ESI.†
CH), 2-(2-pyridyldithio)-ethylamine hydrochloride (PDEA), azido-modified folate are described in ESI.†
Synthesis of polymer precursors
Multivalent HPMA polymer precursor containing thiazolidine-2-thione (TT) reactive groups (P-TT) was prepared by radical solution polymerization as previously described.28 Briefly, HPMA (85 mol%), Ma-ah-NHNH-Boc (5 mol%) and MA-GFLG-TT (10 mol%) were dissolved in dimethylsulfoxide (DMSO) initiated with 2,2′-azobis(isobutyronitrile) (AIBN) (2 wt%). The solution was purged with nitrogen and stirred at 60 °C for 6 h. The polymer was isolated by precipitation into diethyl ether and purified by re-precipitation from methanol into diethyl ether. Boc-protection was used to avoid cross-reaction of the hydrazide groups with TT groups during polymerization.Multivalent HPMA polymer precursor containing amino (NH2) groups (P-NH2) was prepared by aminolysis of TT groups of P-TT with high excess ethylenediamine (avoid branching or crosslinking of P-NH2) in methanol as previously described.16 P-TT (500 mg, 0.24 mmol TT) was dissolved in methanol, and 1.5 mmol ethylenediamine (EDA) was added dropwise. After 1 h of stirring, P-NH2 was purified by gel filtration on a Sephadex LH-20 column using methanol as eluent.
Multivalent HPMA polymer precursor containing thiol (SH) reactive groups (P-SH) was prepared by the reaction of amino groups of P-NH2 with 2-iminothiolane in borate buffer.16 For example, under argon atmosphere, P-NH2 (500 mg, 0.21 mmol amino groups) was dissolved in borate buffer (0.1 M sodium tetraborate, pH 9.6; containing reduced glutathione (GSH) (7 mg mL−1) and a solution of 2-iminothiolane hydrochloride (100 mg, 0.65 mmol) in 3 mL of distilled water was added. After 2 h of stirring, P-SH was purified by gel filtration on a Sephadex G-25 column using double distilled water as eluent, and the polymer precursor was isolated by freeze-drying. The presence of GSH under argon atmosphere was to avoid oxidation and branching.
Semitelechelic polymer precursor (SP-TT) containing alkynyl, tert-butoxycarbonyl (Boc)-protected hydrazide groups and polymer chain terminating with thiazolidine-2-thione groups (TT) was prepared by radical solution polymerization according to the established procedures.29 Briefly, HPMA (90 mol%), Ma-ah-NHNH-Boc (5 mol%) and MA-GG-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH (5 mol%) were dissolved in DMSO initiated with ABIK-TT (4 wt%). The solution was purged with nitrogen and stirred at 50 °C for 6 h. The polymer was isolated by precipitation into diethyl ether and purified by re-precipitation from methanol into diethyl ether.
CH (5 mol%) were dissolved in DMSO initiated with ABIK-TT (4 wt%). The solution was purged with nitrogen and stirred at 50 °C for 6 h. The polymer was isolated by precipitation into diethyl ether and purified by re-precipitation from methanol into diethyl ether.
Similar procedure was followed to prepare fluorescently labeled semitelechelic polymer precursor (SP-TT-FITC), using HPMA (88 mol%), Ma-ah-NHNH-Boc (5 mol%), MA-AP-FITC (2 mol%) and MA-GG-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH (5 mol%).
CH (5 mol%).
The 2-pyridyldisulfanyl (PDS)-terminated semitelechelic HPMA polymer (SP-PDS or SP-PDS-FITC) was prepared by the reaction of terminal TT groups of the polymer SP-TT (or SP-TT-FITC) with 2-(2-pyridyldithio)-ethylamine hydrochloride in N,N-dimethylformamide (DMF) as previously described.30 Briefly, SP-TT (500 mg, 0.048 mmol TT) was dissolved in DMF and a solution of PDEA (14 mg, 0.062 mmol) and N-ethyldiisopropylamine (10 μL) in DMF was added. After 3 h of stirring the reaction mixture was diluted with methanol and purified by gel filtration on a Sephadex LH-20 column using methanol as eluent. The polymer fraction was collected and the polymer was isolated by precipitation into ethyl ether.
Folic acid (FA) was attached to SP-PDS (or SP-PDS-FITC) containing nondegradable dipeptide glycylglycine (GG) spacers by click chemistry. Example of reaction, azido-folate and alkyne-modified SP-PDS were dissolved in 10 mM ammonium bicarbonate. Copper sulfate pentahydrate and a freshly prepared sodium ascorbate solution were added, respectively. The mixture was stirred at room temperature for 24 h. The resulting folate-conjugated SP-PDS was purified by gel filtration on a Sephadex G-25 column using double distilled water as eluent. The polymer solution was lyophilized to obtain the yellow product SP-PDS-FA.
To enable comparison of the activity of biodegradable graft HPMA polymer to that of first generation of low MW linear HPMA polymer (P), a polymer with MW lower than 50 kDa was synthesized by radical polymerization in methanol (AIBN, 2 wt%; molar ratio HPMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ma-ah-NHNH-Boc
Ma-ah-NHNH-Boc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA-GG-C
MA-GG-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH 90
CH 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5; 50 °C; 24 h) as previously described.27 In addition, P-FA, P-FITC and P-FA-FITC were synthesized as described above.
5; 50 °C; 24 h) as previously described.27 In addition, P-FA, P-FITC and P-FA-FITC were synthesized as described above.
Synthesis of folate-decorated graft polymer
The folate targeted high MW graft polymer (GP-FA) was prepared by grafting semitelechelic polymer SP-PDS-FA, bearing reactive PDS end groups, onto polymer P-SH containing thiol reactive groups in the side chains. Folate modified semitelechelic polymer SP-PDS-FA (260 mg) was dissolved in phosphate buffer (0.05 M sodium dihydrogen phosphate/disodium hydrogen phosphate, 0.1 M sodium chloride, 0.01 M ethylenediaminetetraacetic acid (EDTA), pH 7.4), a solution of P-SH (100 mg, 0.035 mmol SH groups) in 1 mL of phosphate buffer was added and stirred for 1 h. The reaction mixture was diluted with double distilled water and purified by gel filtration on a Sephadex G-25 column using double distilled water as eluent. The final folate-targeted graft polymer was obtained by freeze-drying.Similar procedure was followed to prepare non-targeted graft polymer GP (consisted of SP-PDS and P-SH), fluorescently labeled graft polymer GP-FITC (consisted of SP-PDS-FITC and P-SH) and fluorescently labeled folate targeted graft polymer GP-FA-FITC (consisted of SP-PDS-FA-FITC and P-SH).
The near-infrared dye Cy5.5 labeled folate targeted graft polymer (GP-FA-Cy5.5) was synthesized after removing the protective Boc groups from the hydrazides in graft polymer with concentrated trifluoroacetic acid. Then Cy5.5-NHS ester (2 mg, 2.79 μmol) in DMSO was slowly dropped into GP-FA (50 mg) in sodium bicarbonate solution (0.1 M, pH 8.5), and N-ethyldiisopropylamine (10 μL) was added. The reaction was performed at room temperature in the dark overnight. Unreacted Cy5.5 molecules were removed by gel filtration on a Sephadex G-25 column using double distilled water as eluent and the resulting product was lyophilized.
Characterization of polymers
The content of end-chain TT groups was determined by UV-vis spectroscopy using ε305 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 L mol−1 cm−1 (methanol). The content of PDS end groups was determined by UV-vis spectroscopy after reaction with dithiothreitol.15 The content of Gly–Gly sequences was determined by amino acid analysis (Commonwealth Biotech, VA, USA). The yield of the conjugation with folate was determined by UV-vis spectroscopy using ε281 = 20
700 L mol−1 cm−1 (methanol). The content of PDS end groups was determined by UV-vis spectroscopy after reaction with dithiothreitol.15 The content of Gly–Gly sequences was determined by amino acid analysis (Commonwealth Biotech, VA, USA). The yield of the conjugation with folate was determined by UV-vis spectroscopy using ε281 = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 L mol−1 cm−1 (0.1 M borate buffer, pH 9.0). The content of amino (NH2) or hydrazide groups was determined by TNBSA assay. The content of SH groups in polymer precursors was determined with Ellman's reagent.31 The content of FITC in polymers was determined by UV-vis spectrometry using ε494 = 80
650 L mol−1 cm−1 (0.1 M borate buffer, pH 9.0). The content of amino (NH2) or hydrazide groups was determined by TNBSA assay. The content of SH groups in polymer precursors was determined with Ellman's reagent.31 The content of FITC in polymers was determined by UV-vis spectrometry using ε494 = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L mol−1 cm−1 (0.1 M borate buffer, pH 9.0). The conjugation ratios of Cy5.5 to polymer was determined by measuring fluorescence intensity (λex = 676 nm, λem = 707 nm) in DMSO.
000 L mol−1 cm−1 (0.1 M borate buffer, pH 9.0). The conjugation ratios of Cy5.5 to polymer was determined by measuring fluorescence intensity (λex = 676 nm, λem = 707 nm) in DMSO.
The MW and polydispersity index (PDI) of polymers were measured on a GPC/HPLC system (Agilent Technologies 1200 series) equipped with refractive index, UV and multiangle light scattering DAWN HELEOS II (Wyatt Technology Co.) detectors using phosphate buffer (pH 7.4) and Superose™ 6 column.
The size distribution [hydrodynamic radius (Rh)] and zeta potential of folate-modified polymers in deionized water (concentration 1 mg mL−1) were measured on a Malvern Zetasize NanoZS90 instrument (Malvern Instruments Ltd., Malvern, UK). The morphologies of P-FA, GP-FA and degradation products of GP-FA were observed by a transmission electron microscopy (FEI Tecnai GF20S-TWIN, USA).
In vitro degradation of graft polymers
Graft polymer GP-FA and GP were incubated in McIlvaine's buffer (50 mM citrate/0.1 M phosphate, 2 mM EDTA, pH 6.0) at 37 °C in the presence of papain (8 mg mL−1) and reduced glutathione (GSH) (3 mM) with concentration of polymer 10 mg mL−1 for 24 h. At predetermined time points, a sample was withdrawn and the MW was tested by gel permeation chromatography (GPC).Cell culture
The folate-receptor overexpressed MCF-7 human breast adenocarcinoma cell line32 was purchased from Chinese Academy of Science Cell Bank for Type Culture Collection (Shanghai, China), and was cultured in DMEM/F12 medium (Gibco) supplemented with 10% fetal bovine serum (Hyclone) and 1% penicillin–streptomycin (Hyclone) at 37 °C in 5% CO2 atmosphere. All experiments were performed on cells in the logarithmic growth phase.Endocytic uptake analysis
For studies of cell uptake by flow cytometry, MCF-7 cells were cultured in 12-well plates (Corning, USA) at a density of 1 × 105 cells per well and incubated for 24 h. Then the cells were incubated with P-FITC, P-FA-FITC, GP-FITC and GP-FA-FITC at 37 °C for the indicated time periods. After incubation, medium was removed. The cells were harvested and washed with PBS for three times followed immediately by flow cytometry analysis (Cytomics™ FC 500, Beckman Coulter Ltd.). The amount of 1.0 × 104 cells was collected, and the mean fluorescence intensity was recorded for each sample.For confocal studies, cells were grown on sterile cover glasses in a 6-well plate at a concentration of 1 × 105 cells per well and incubated for 24 h. The medium was then replaced with medium containing P-FITC, P-FA-FITC, GP-FITC and GP-FA-FITC (0.4 mg polymer mL−1). After 2 h of incubation, cells were washed with PBS, fixed using 4% paraformaldehyde solution in PBS, DAPI stained, and washed again with PBS. The cover glasses were mounted on the glass slides with a drop of anti-fade mounting media and visualized under a confocal laser scanning microscope (LSM 510 DUE, Carl Zeiss, Jena, Germany).
Study of cellular uptake mechanisms
With the aim of investigating whether the multimer effect or different size was responsible for the cellular uptake, folate-decorated graft polymers GP-FA-FITC-1 and non-targeted graft polymers GP-FITC-1 with smaller Rh in comparison with GP-FITC were prepared. Then the MCF-7 cells were incubated with P-FITC, P-FA-FITC, GP-FITC-1, GP-FITC, GP-FA-FITC-1 and GP-FA-FITC for 2 h at 37 °C, the intracellular internalization of various formulations was processed by flow cytometry as described above.In order to study the possible internalization pathway of low MW linear and high MW graft HPMA polymers on MCF-7 cells. The cells were precultured with clathrin endocytosis inhibitor (30 μM chlorpromazine), caveolae endocytosis inhibitor (5 μM filipin), macropinocytosis inhibitor (60 μM wortmannin, 10 μM rottlerin) and 2 mM folic acid. Alternatively, cells were incubated at 4 °C. After 1 h pretreatment, the cells were re-incubated with P-FITC, P-FA-FITC, GP-FITC and GP-FA-FITC (0.4 mg polymer mL−1) at the same conditions for 2 h. Next, the cells were washed with PBS and then processed for flow cytometry as described above. In addition, the control sample of each group was incubated with indicated polymer solution at 37 °C for 2 h without any treatment. The results of the inhibition tests were presented as the percentage of that internalized in control.
To test the binding efficiency of folate attached to linear and graft HPMA polymers, indirect immunofluorescence by flow cytometry was performed. First, MCF-7 cells were pre-incubated with P, P-FA, GP and GP-FA (0.4 mg polymer mL−1) for 1 h at 37 °C, and then the cells were washed with PBS and gently removed from plates by trypsination. Cells were then resuspended and incubated with primary antibody MAb MOv-18 (dilution = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) in a total volume of 80 μL blocking solution at 4 °C in dark for 1.5 h to compete for binding the folate receptor. Then the solution was removed by centrifugation and the cells were washed by cold BSA (3%) containing PBS solution. 100 μL of the FITC-conjugated goat anti-rabbit IgG (H+L) secondary antibody (dilution = 1
20) in a total volume of 80 μL blocking solution at 4 °C in dark for 1.5 h to compete for binding the folate receptor. Then the solution was removed by centrifugation and the cells were washed by cold BSA (3%) containing PBS solution. 100 μL of the FITC-conjugated goat anti-rabbit IgG (H+L) secondary antibody (dilution = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) in PBS was added and further incubated for 30 min. Next, cells were washed with PBS and then processed for flow cytometric analysis immediately.
40) in PBS was added and further incubated for 30 min. Next, cells were washed with PBS and then processed for flow cytometric analysis immediately.
Cytotoxicity assay
Cytotoxicity was assessed using a tetrazolium dye (MTT) assay based on the decrease of MTT formazan crystals by living cells. MCF-7 cells were seeded on 96-well plates at a density of 1 × 104 cells per well, and cultured for 24 h. Subsequently, the cells were exposed to polymers (P, P-FA, GP and GP-FA) dispersed in DMEM/F12 medium at different concentrations (0.15, 0.43, 0.72, 0.86 and 1 mg mL−1). After 48 h of incubation, the cells were incubated with MTT dye for another 4 h. Then the medium was removed before adding 150 μL of DMSO to each well to dissolve the formazan precipitate, and absorbance was measured at 570 nm using a plate reader (Thermo, Varioskan Flash). Untreated cells were used as control, and the viability was expressed as the percentage of the absorbance of control group.Animals
Female SD rats (200 ± 20 g) and female BALB/c nude mice (5–8 weeks old and weighted 18–20 g) were purchased from Animal Centre of the Institute of West China Medical Center and all animals received care in compliance with the approved guidelines by the animal welfare committee at Sichuan University and were provided with food and water. All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals.Pharmacokinetics study
For pharmacokinetic study, healthy female SD rats (n = 5) were injected intravenously with P-Cy5.5, P-FA-Cy5.5, GP-Cy5.5 and GP-FA-Cy5.5, respectively, at Cy5.5 dose of 1.5 mg kg−1. At predetermined intervals, blood samples (300 μL) were taken from the tail vein. Plasma samples were harvested by immediate centrifugation at 800 × g for 10 min and store at −20 °C for analysis. To precipitate plasma protein, 300 μL methanol was added to 100 μL plasma, followed by vortex for 1 min standing at −20 °C for 30 min, and centrifugation at 8910 × g for 5 min. After that, 150 μL of the supernatant was added to a black 96-well plate and fluorescence intensity (Ex/Em: 673/692) was measured using a microplate reader (Thermo, Varioskan Flash). The total volume of blood was assumed to be 7% of body weight.33 The percent injected dose (%ID) was calculated, and the blood pharmacokinetics parameters were analyzed using a noncompartmental model with DAS 2.0.1 (Bojia, Shanghai, China).In vivo imaging and biodistribution analysis
To establish human breast cancer xenografts, MCF-7 cells (6 × 106 cells/100 μL) were administered by subcutaneous injected into the right flank of the mice. When the tumor volumes reached about 300–400 mm3, the mice were divided into four groups (n = 3). P-Cy5.5, P-FA-Cy5.5, GP-Cy5.5 or GP-FA-Cy5.5 (equivalent to 1.5 mg kg−1 Cy5.5) were injected to nude mice via tail vein. Images were taken at 1, 2, 3, 4 and 5 days after injection using the Bio-Real Quick View 3000 (Geneway International, Austria) in vivo imaging system.To compare the tissue and tumor distributions of the polymers, after 48 h post-injection of P-Cy5.5, P-FA-Cy5.5, GP-Cy5.5 and GP-FA-Cy5.5 (equivalent to 1.5 mg kg−1 Cy5.5), the mice were sacrificed (n = 3). The major organs including heart, lung, liver, kidneys, brain, spleen and tumor were dissected, which were then washed with normal saline and imaged immediately for semiquantitative analysis by an in vivo imaging system as mentioned above.
Statistical analysis
Data are presented as mean ± SD. Statistical analyses of data were done using Student's t-test. Significant differences were defined as p < 0.05, and highly significant p < 0.01.Results and discussion
Synthesis and characterization of polymer precursors and graft polymers
Several kinds of high MW HPMA polymer drug carriers, such as branched, star and graft HPMA polymers, have shown increased tumor accumulation by enhanced EPR effect.15–17 Regrettably, few works about the ligand modified high MW HPMA polymers have been reported. With the aim to gain some insight of the impact of hydrophilic polymer structure on the targeting efficiency of the conjugated ligand, in the present study, folate modified graft HPMA polymers were synthesized by grafting several similar but semitelechelic HPMA polymer precursors onto a main multivalent HPMA polymer chain through enzymatically degradable oligopeptide glycylphenylalanylleucylglycine sequence and reductively degradable disulfide bonds (GFLG-S-S) as shown in Fig. 1. Table 1 summarizes key polymer characteristics such as MW, PDI, reactive group content.| Polymer | MW (kDa) | PDI | Reactive group (mmol g−1 polymer) | FITC content (mmol g−1 polymer) | Folate content (mmol g−1 polymer) | Cy5.5 content (wt%) |
|---|---|---|---|---|---|---|
| SP-TT | 29.4 | 1.54 | TT (0.096) | — | — | — |
| SP-TT-FITC | 32.2 | 1.67 | TT (0.091) | 0.073 | — | — |
| SP-PDS | 30.7 | 1.62 | PDS (0.077) | — | — | — |
| SP-PDS-FITC | 33.6 | 1.86 | PDS (0.073) | 0.071 | — | — |
| SP-PDS-FA | 32.5 | 1.75 | PDS (0.071) | — | 0.18 | — |
| SP-PDS-FA-FITC | 35.4 | 1.84 | PDS (0.069) | 0.069 | 0.16 | — |
| P-TT | 20.1 | 1.26 | TT (0.48) | — | — | — |
| P-NH2 | 18.9 | 1.32 | NH2 (0.42) | — | — | — |
| P-SH | 18.5 | 1.47 | SH (0.35) | — | — | — |
| P | 25.7 | 1.81 | — | — | — | — |
| P-FITC | 24.5 | 1.77 | — | 0.075 | — | — |
| P-FA | 27.3 | 1.86 | — | — | 0.19 | — |
| P-FA-FITC | 27.9 | 1.95 | — | 0.073 | 0.18 | — |
| P-Cy5.5 | 24.8 | 1.83 | — | — | — | 2.8 |
| P-FA-Cy5.5 | 27.6 | 1.92 | — | — | 0.17 | 2.7 |
| GP | 106.6 | 2.74 | — | — | — | — |
| GP-FITC | 112.5 | 2.86 | — | 0.049 | — | — |
| GP-FA | 117.4 | 3.06 | — | — | 0.11 | — |
| GP-FITC-FA | 119.7 | 3.35 | — | 0.052 | 0.12 | — |
| GP-Cy5.5 | 107.3 | 2.79 | — | — | — | 2.6 |
| GP-FA-Cy5.5 | 117.6 | 3.13 | — | — | 0.11 | 2.8 |
Due to the difficulty of direct synthesis of polymerizable oligopeptide containing thiol group monomers, the multivalent polymer precursor with thiol groups randomly distributed along the polymer chain (P-SH) was prepared in three steps. Firstly, the polymer precursor (P-TT) was synthesized by using a polymerizable oligopeptide containing TT reactive group monomer (MA-GFLG-TT). The molar ratio of monomers used for polymerization in the main polymer chain P-TT was HPMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ma-ah-NHNH-Boc
Ma-ah-NHNH-Boc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA-GFLG-TT = 85
MA-GFLG-TT = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The high content of HPMA in the polymers (>80 mol%) would guarantee the water-solubility and biocompatibility of the carriers.34 MA-GFLG-TT content was determined from the amount of TT groups and the content was 0.48 mmol g−1 polymer. The content of Ma-ah-NHNH-Boc was 4.12 mol%. In contrast to routinely used polymer precursor bearing 4-nitrophenyl ester groups (ONp) in the side chains, thiazolidine-2-thione reactive groups (TT) were introduced in this study, which associated with a higher rate of aminolysis and a much slower rate of hydrolysis during aminolysis performed in aqueous medium.28 Then polymer precursor bearing reactive amino groups (P-NH2, amimo content = 0.42 mmol g−1 polymer) was synthesized by aminolysis of the TT groups with ethylenediamine (EDA) since the primary amino groups of P-NH2 were essential to generate thiol groups. Accordingly, polymer precursor bearing reactive thiol groups (P-SH, thiol content = 0.35 mmol g−1 polymer) was prepared by the reaction of P-NH2 with 2-iminothiolane (a thiolating reagent for primary amines). The thiol reactive groups of P-SH could react with pyridyldisulfanyl (PDS) reactive groups of semitelechelic polymer SP-PDS to form graft polymer via both enzymatically degradable and reductively degradable linkage GFLG-S-S.
10. The high content of HPMA in the polymers (>80 mol%) would guarantee the water-solubility and biocompatibility of the carriers.34 MA-GFLG-TT content was determined from the amount of TT groups and the content was 0.48 mmol g−1 polymer. The content of Ma-ah-NHNH-Boc was 4.12 mol%. In contrast to routinely used polymer precursor bearing 4-nitrophenyl ester groups (ONp) in the side chains, thiazolidine-2-thione reactive groups (TT) were introduced in this study, which associated with a higher rate of aminolysis and a much slower rate of hydrolysis during aminolysis performed in aqueous medium.28 Then polymer precursor bearing reactive amino groups (P-NH2, amimo content = 0.42 mmol g−1 polymer) was synthesized by aminolysis of the TT groups with ethylenediamine (EDA) since the primary amino groups of P-NH2 were essential to generate thiol groups. Accordingly, polymer precursor bearing reactive thiol groups (P-SH, thiol content = 0.35 mmol g−1 polymer) was prepared by the reaction of P-NH2 with 2-iminothiolane (a thiolating reagent for primary amines). The thiol reactive groups of P-SH could react with pyridyldisulfanyl (PDS) reactive groups of semitelechelic polymer SP-PDS to form graft polymer via both enzymatically degradable and reductively degradable linkage GFLG-S-S.
Meanwhile, PDS-terminated semitelechelic HPMA polymer precursor (SP-PDS) was synthesized according to Fig. 1. In the first step, solution radical polymerization of HPMA with respective monomers was initiated with ABIK-TT. This bifunctional azoinitiator, which contained reactive TT groups, was used for the synthesis of semitelechelic HPMA polymer terminating with TT groups (SP-TT).29 The molar ratio of monomers used for polymerization in the semitelechelic polymer precursor SP-TT was HPMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ma-ah-NHNH-Boc
Ma-ah-NHNH-Boc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA-GG-C
MA-GG-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH = 90
CH = 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. The content of Ma-ah-NHNH-Boc was 4.16 mol%. MA-GG-C
5. The content of Ma-ah-NHNH-Boc was 4.16 mol%. MA-GG-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH content was determined from the amount of GG sequences and the content was 4.35 mol%. Secondly, the semitelechelic polymer bearing an end-chain reactive PDS groups (SP-PDS, PDS content = 0.077 mmol g−1 polymer) was prepared by aminolysis of SP-TT with 2-(2-pyridyldisulfanyl)-ethylamine hydrochloride (PDEA). The end-chain reactive PDS groups served to graft onto the thiol reactive groups of P-SH.
CH content was determined from the amount of GG sequences and the content was 4.35 mol%. Secondly, the semitelechelic polymer bearing an end-chain reactive PDS groups (SP-PDS, PDS content = 0.077 mmol g−1 polymer) was prepared by aminolysis of SP-TT with 2-(2-pyridyldisulfanyl)-ethylamine hydrochloride (PDEA). The end-chain reactive PDS groups served to graft onto the thiol reactive groups of P-SH.
In terms of ligand decoration, in most cases the strategy for polymer–ligand conjugate synthesis is based on aminolysis of a polymer precursor bearing reactive esters (ONp or TT) in the side chains with amino group of ligand. However, in this case, as reactive TT groups already existed on the terminal of semitelechelic HPMA polymer precursor (SP-TT), the introduction of reactive esters in the side chains of SP-TT for the attachment of folate might give rise to the cross-reaction of PDEA. To ensure feasibility of folate conjugation, “click chemistry” was brought in, instead of traditional amine-carboxyl reaction. Click chemistry with its unique features such as high aqueous competency and chemical orthogonality, has emerged as a burgeoning strategy for the development of surface-engineered polymers and nanoparticles with high selectivity and efficiency.35 Considering significant loss of azide moiety in polymerization of various monomer,36 alkyne modified monomer MA-GG-C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH was selected to “click” with azide-modified folate. As shown in Table 1, the MW of all the polymer precursors were under the renal threshold, which was a prerequisite for subsequent elimination of the polymers from the body.
CH was selected to “click” with azide-modified folate. As shown in Table 1, the MW of all the polymer precursors were under the renal threshold, which was a prerequisite for subsequent elimination of the polymers from the body.
Ultimately, the folate-conjugated graft polymers (GP-FA) were prepared by grafting folate-modified PDS-terminated semitelechelic polymers (SP-PDS-FA) onto the main polymer chain of P-SH with thiol reactive groups randomly distributed along the polymer chain via both reductively degradable disulfide bonds and enzymatically degradable oligopeptide glycylphenylalanylleucylglycine sequence (GFLG-S-S, Fig. 1). The free hydrazide groups were deprotected of Boc-hydrazides for the attachment of Cy5.5. Table 1 showed the MW of the graft polymers was approximately 110 kDa, indicating that, on average, three semitelechelic polymer chains (MW: 20–30 kDa) were attached to the main HPMA polymer chain. The polydispersity of the graft polymers was higher than that of the linear polymer, but was significantly narrower than that of the previously reported branched high MW HPMA polymers.15
The increased MW by grafting several semitelechelic HPMA polymer chains and modification of negatively charged folate ligand may trigger changes in architecture and potential of graft polymer. As confirmed in Fig. 2A, the hydrodynamic radius (Rh) of the high MW graft polymer coil in aqueous solution was 16.1 nm with the zeta potential of −7.2 mV, which was 3 times larger than that of low MW linear polymers (5.6 nm) with the zeta potential of 1.5 mV. TEM results also showed that Rh of GP-FA coil was larger than that of P-FA, which was consistent with Rh obtained by DLS (Fig. 3C). This was the prerequisite for the enhanced tumor accumulation of polymers due to the more pronounced EPR effect.
Polymer carriers with MW above the renal threshold (∼50 kDa) will deposit in normal organs and impair biocompatibility,23 hence, the biodegradation of polymers to smaller fragments is imperative. Herein, we studied the in vitro degradation of graft polymers. The reductive degradation of disulfide bonds (S–S) of polymers by intracellular glutathione (GSH) has been widely investigated.37 GSH is a thiol-containing tripeptide and could reduce disulfide bonds in the cytoplasm. The intracellular concentration of GSH was known to be substantially higher than that in the cell exterior. Also, an elevated intracellular GSH level has been reported in many human tumor cell lines.38 Meanwhile, an optimal structure of enzyme sensitive oligopeptide (glycylphenylalanylleucylglycine, GFLG) spacer, which is stable in blood and intracellularly degradable,39 was widely used for drug conjugation in HPMA polymers. Above all, in the present study, the combined application of GFLG-S-S was selected as the spacer to link the HPMA precursors, which guarantees the stability of high MW folate-conjugated graft polymers during transport and enable their intracellular degradation to short polymer fragments allowing safe elimination from body, e.g. by glomerular filtration.
To test the degradability of folate-conjugated graft polymer, GP-FA was incubated in phosphate buffer (pH 6.0) containing both lysosomal cysteine protease papain (which cleaves GFLG linkages)40 and GSH (modeling cytosolic environment) to simulate the intracellular environment. As expected, within 3 h of incubation, GP-FA was completely degraded to polymer fragments with MW (∼42 kDa) below the renal threshold (∼50 kDa) (Fig. 2B). Then the polymer fragments kept on degradation to an analogous MW of 30.7 kDa with the control sample (PDS-terminated semitelechelic HPMA polymer SP-PDS) at 6 h. The MW of degradation product at 8 h was smaller than that of P-SH (18.5 kDa). TEM images showed that Rh of degradation products of GP-FA was decreased in comparison with GP-FA in line with GPC profiles (Fig. 3C). This demonstrated the ability of GP-FA to be degraded to the polymer fragments below the renal threshold after being internalized by tumor cells and capable of being excreted from body by glomerular filtration. The degradation rate of GP was comparable with GP-FA (Fig. S5†), indicating that incorporation of folate to graft polymers did not result in alteration of their degradation rate.
In summary, the high MW was obtained by graft polymers GP-FA (117.4 kDa) compared with low MW linear polymers P-FA (27.3 kDa). In addition, GP-FA could be degraded into smaller fragments in the tumor cells, thus reducing the potential toxicity of the carrier.
Endocytic uptake
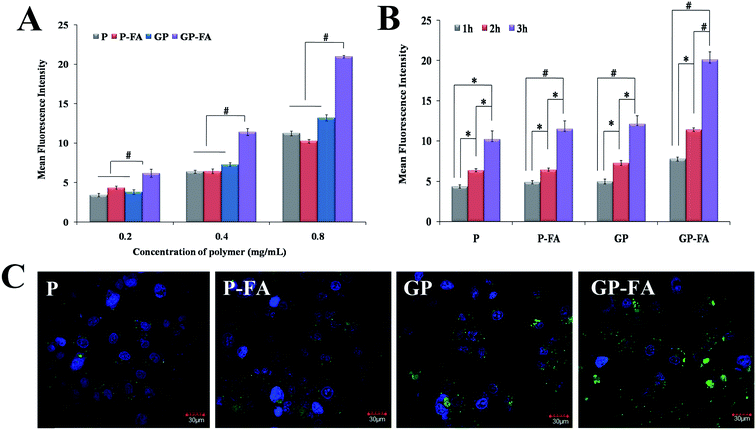
To further compare the different roles of high MW graft polymers and low MW linear polymers played in folate targeting capability to promote the intracellular internalization, we performed flow cytometry (Fig. 3A and B) and confocal microscopy (Fig. 3C) in folate-receptor positive MCF-7 cell line using FITC-labeled HPMA polymers (P, P-FA, GP and GP-FA) as test samples.As shown in Fig. 3A, the uptake of all polymers was concentration-dependent (from 0.2 mg polymer mL−1 to 0.8 mg polymer mL−1). Significantly higher fluorescence intensity (∼2-fold) was observed on GP-FA group, compared to P, P-FA and GP (p < 0.01). Besides, the intracellular internalization of all polymers elevated with prolonged incubation time (1 h to 3 h, Fig. 3B). In agreement with the results of flow cytometry, confocal images also exhibited the highest uptake of GP-FA compared with other test polymers (Fig. 3C). It was interesting that no significant difference was observed between the uptake of P and GP, demonstrating that the different MW (24.5 kDa to 119.7 kDa) has no effect on the cell internalization of non-targeted HPMA polymers. Conversely, different phenomenon was observed for folate modified polymers. For low MW linear polymers, P-FA showed comparable internalization with P while graft GP-FA exhibited significantly higher uptake over GP (2-fold higher). Inaccessibility to folate receptor by hydrophobic folate buried inside the hydrophilic linear polymer chains may possibly be responsible for the lack of targeting efficiency of P-FA. This is supported by several reports that used different drug carriers (e.g., PEG polymers11 and PLGA nanoparticles12). Although the negatively charged particles could minimize the cell uptake due to the charge repulsion, GP-FA (−7.2 mV) still exhibited enhanced intracellular internalization in comparison with neutral charge of P-FA (1.5 mV). This may attribute to the higher exposure of negatively charged folate on the surface of GP-FA can overcome the negative impact of its negative charge, and contribute to the increased internalization via folate receptor-mediated endocytosis.
Cellular uptake mechanisms
In order to investigate whether the enhancement of internalization purely a result of the multimer effect or the size needed, the cellular uptake of folate modified polymers (P-FA, Rh = 5.6 nm; GP-FA-1, Rh = 10.2 nm; GP-FA, Rh = 16.1 nm) and non-targeted polymers (P, Rh = 5.1 nm; GP-1, Rh = 11.4 nm; GP, Rh = 15.2 nm) with different Rh was studied. The conjugated folate content of P-FA, GP-FA-1 and GP-FA were 0.18 mmol g−1 polymer, 0.14 mmol g−1 polymer and 0.12 mmol g−1 polymer, respectively. As shown in Fig. 4A, no significant difference was observed among the uptake of P (5.1 nm), GP-1 (11.4 nm) and GP (15.2 nm), demonstrating that the multimer effect and size had negligible effect on the cell internalization of non-targeted HPMA polymers. On the contrary, different phenomenon was observed for folate modified polymers (Fig. 4B). The internalization of both graft polymers GP-FA-1 and GP-FA increased when compared with linear P-FA, indicating the multimer effect played some role in the enhancement of folate binding efficiency for graft polymers. In addition, stronger endocytosis was observed in GP-FA with larger size (16.1 nm) than GP-FA-1 with smaller size (10.2 nm), demonstrating that increasing the size could also favor the enhanced binding efficiency of folate modified graft polymers to some extent. Collectively, as multimer structure and polymer size are highly correlated and could greatly influence each other, they may all have crucial impact on the folates' position in the polymers. As reflected by the lower negative charge of GP-FA (−7.2 mV) in comparison with GP-FA-1 (−3.5 mV) and P-FA (1.5 mV), suggesting the graft polymers with larger size possessed increased amount of folate exposure than its smaller counterpart and linear one. Therefore, the multimer effect and increased size of folate targeted graft polymers both contributed to the enhancement of internalization.In order to study the possible internalization pathway of folate modified low MW linear and high MW graft HPMA polymers on MCF-7 cells. Then the cellular uptake mechanisms of linear and graft polymers were investigated under different conditions. To verify the specific role of folate, the effects of free folate on the uptake of the polymers were investigated. As shown in Fig. 4C, a more remarkable decrease was observed for GP-FA (∼38%), while the other test samples including folate modified linear polymers (P-FA) remained uninfluenced after co-incubation with free folate (p < 0.01), suggesting that the internalization of GP-FA in MCF-7 cells indeed involved folate receptor-mediated endocytosis. When the experiments were performed at 4 °C, all polymers exhibited a very low uptake level (∼70%, p < 0.01). These results clearly underlined that all the test samples enter the cells following an energy-dependent endocytosis. Caveolae-mediated endocytosis is an important pathway for cellular internalization of drugs, which can be selectively inhibited by filipin through cholesterol sequestration.41 The cellular uptake of GP-FA was inhibited approximately 30% by filipin, in line with some folate modified drug delivery systems that were mainly uptaken by caveolae-mediated endocytosis.42 A significant inhibition (∼27%, p < 0.05) of P-FA, GP and GP-FA was observed when cells were pretreated with chlorpromazine, which can block clathrin-mediated endocytosis by disruption of clathrin and other relative proteins expressed on cell membrane,43 suggesting the involvement of clathrin-mediated endocytosis. To investigate whether the macropinocytosis was involved in the internalization pathways of graft and linear polymers, wortmannin and rottlerin were used as chemical inhibitors. The former could inhibit phosphatidylinositol-3-kinase (PI3K) during the subpathway of macropinocytosis44 while the latter is a novel macropinocytosis inhibitor through inhibiting protein kinase C irreversibly and selectively.45 As shown in Fig. 4C, no significant decrease of cellular uptake was observed for all test polymers after incubation with wortmannin while apparent decreased intracellular internalization occurred with the treatment of rottlerin, indicating the protein kinase C mediated pathway was more involved in macropinocytosis rather than PI3K-mediated pathway. Notably, more significantly decreased intracellular fluorescence intensity was detected in GP and GP-FA groups (∼35%, p < 0.01) by rottlerin. As mentioned above, the Rh of graft HPMA polymers (16.1 nm) was much higher than that of linear one (5.6 nm). To some degree, the larger size of graft polymer coil might result in more cellular uptake by macropinocytosis with the aid of protein kinase C.
Then the binding affinity of folate attached to the linear and graft polymers were also investigated. As shown in Fig. 5, the primary antibody alone bound to 98.1% of tested cells, leading to the strongest fluorescence (10.67), while the percentage of antibody-bounded cell significantly decreased to 3.5% with the addition of free folate, demonstrating the competitive binding of free folate to folate-receptor positive MCF-7 cells. Notably, compared with P-FA, the fluorescence intensity of secondary antibody reduced significantly from 9.26 to 2.67 after the incubation with GP-FA (p < 0.01), thus confirming that the folate binding affinity of graft polymers was enlarged in competition with that of linear polymers.
These results implied that folate receptor-mediated endocytosis, energy-dependent endocytosis, caveolae-mediated endocytosis, clathrin-mediated endocytosis and protein kinase C-mediated macropinocytosis were vigorously engaged in the internalization pathways of GP-FA, indicating that relatively high contribution of each pathways results in overall the highest uptake.
Cytotoxicity assay
MTT assay was performed to investigate the cytotoxicity of P, P-FA, GP and GP-FA against MCF-7 cells (Fig. 6). Cells were incubated with different polymers for 48 h. At every studied concentration (from 0.15 mg polymer mL−1 to 1 mg polymer mL−1), the viability of MCF-7 cells was above 90% for all polymers, and no significant difference was found among all of the test polymers, indicating their uniformly low cytotoxicity, further demonstrating their potential as excellent drug carriers. | ||
| Fig. 6 Cell viability of MCF-7 cells after 48 h of incubation with various concentrations of P, P-FA, GP and GP-FA. Data are presented as the mean ± SD (n = 3). | ||
Pharmacokinetics study
It is important to keep in mind in this regard that the passive targeting mainly relies on the prolonged circulation time of nanocarriers. The active targeting by incorporation of ligands could result in increased cell internalization in vitro, however, it also generally has deleterious pharmacokinetic consequences.46 The effect of folate on the pharmacokinetics of low MW linear polymers and high MW graft polymers needed to be clarified.The blood (fluorescence) activity–time profiles in SD rats after intravenous injection of near-infrared dye (Cy5.5)-labeled polymers (P, P-FA, GP and GP-FA) are presented in Fig. 7. The blood levels of low MW linear polymers (P and P-FA) fell rapidly to nearly 25% of the injected dose within 3 h post-injection, whereas prolonged blood residence time of high MW graft polymers (GP and GP-FA) were observed, thus demonstrating the MW dominated the circulation time of drug carriers, rather than ligand modification. Pharmacokinetic parameters, which were estimated by noncompartmental analysis of the blood concentration (%ID per mL), were summarized in Table 2. The biological half-life (t1/2) of GP and GP-FA (21.28 h and 19.14 h) were higher than that of P and P-FA (7.65 h and 9.08 h). High MW polymers had significantly higher area under the blood concentration time curve (AUC) in comparison to P and P-FA (p < 0.01). In addition, the mean systemic clearance (CL) was significantly lower in GP as compared to P (0.084 mL h−1 vs. 0.23 mL h−1, p < 0.01), as well as the mean residence time (MRT) of GP was also significantly longer than that of P (28.98 h vs. 10.56 h, p < 0.01). As a consequence, graft polymers with relatively high MW were eliminated more slowly from the blood than P and P-FA with low MW, demonstrating that increased MW can prolong blood circulation time in vivo.
| Polymer | t1/2 (h) | AUC0–∞ (%ID h mL−1) | CL (mL h−1) | MRT (h) |
|---|---|---|---|---|
| a Data are presented as mean ± SD (n = 5, *p < 0.05 vs. P-FA, #p < 0.01 vs. GP, △p < 0.05,&p < 0.01 vs. GP-FA). | ||||
| P | 7.65 ± 1.01#& | 403.47 ± 28.44*#& | 0.23 ± 0.03*#& | 10.56 ± 1.92#& |
| P-FA | 9.08 ± 1.37#& | 508.54 ± 43.41#& | 0.18 ± 0.02#& | 11.42 ± 1.42#& |
| GP | 21.28 ± 3.27 | 1516.77 ± 52.33△ | 0.084 ± 0.01 | 28.96 ± 3.78 |
| GP-FA | 19.14 ± 2.45 | 1201.45 ± 64.78 | 0.12 ± 0.02 | 27.37 ± 2.59 |
The t1/2 and MRT of folate targeted polymers were not significantly different from non-targeted polymers. Nevertheless, the CL of P was significantly higher than P-FA (0.23 mL h−1 vs. 0.18 mL h−1, p < 0.05). The negative surface charge of P (−10.7 mV) may contribute to its fast elimination from blood circulation in comparison to near neutral P-FA (1.5 mV).23 Last but not least, the higher exposure level of folate on graft polymers (GP-FA) caused slight increased blood clearance. The decreased water solubility of the polymers and promoted recognition by macrophages might probably account for this limitation.13 Even so, the blood circulation time of GP-FA was still much longer than that of P and P-FA.
Imaging and biodistribution analysis
It has become increasingly clear that a nanodevice which exhibits superior in vitro tumor-homing capability is not predictive of in vivo results. To further investigate the impact of HPMA polymer structure on the in vivo distribution of folate decorated polymers, P-Cy5.5, P-FA-Cy5.5, GP-Cy5.5 and GP-FA-Cy5.5 were injected intravenously through the tail vein (equivalent to 1.5 mg kg−1 Cy5.5) to mice bearing MCF-7 tumor xenografts. Fig. 8A showed that Cy5.5-labeled GP and GP-FA had significantly stronger fluorescent intensity in the whole body over the entire study period than P and P-FA, in agreement with the results of lower blood clearance observed in pharmacokinetic study. Evident fluorescence intensity was observed in kidney at 1 day after injection, demonstrating the renal clearance of the tested HPMA polymers.47 By day 3, the whole-body fluorescent signals in mice treated with low MW linear polymers P were much lower than those treated with high MW graft polymers GP, demonstrating once again the long retention time in circulation of GP. Moreover, the fluorescence intensity of GP and GP-FA also decreased from day 1 to day 5, indicating that account for higher level of glutathione and lysosomal protease in tumor cells, intracellular enzymatic and reductive degradation of graft polymers were occurred. This further documented that the biodegradable polymers can temporarily serve as drug carriers to deliver drugs to the tumor sites, and then be broken down spontaneously in a predetermined manner into segments eliminated from the body. It was observed that GP and GP-FA had significantly higher fluorescence intensity in tumor than P and P-FA, especially from day 2, demonstrating the enhanced tumor accumulation by the contribution of increased MW. It should be noted that the tumor accumulation of GP-FA was higher than that of GP, indicating that enhanced EPR effect and ligand–receptor interaction delayed the clearance of polymer from tumor tissues.At 48 hours after injection, mice were sacrificed. The major organs including heart, liver, spleen, lung, kidneys, brain and tumor were collected, and all tissues were analyzed by fluorescence imaging (Fig. 8B). Stronger fluorescence intensity in tumor and kidney was observed for GP-FA than other test polymers. As shown in Fig. 8C, the fluorescence intensity of tumor tissue of GP-FA was significantly higher than all organs except kidneys. Furthermore, when P was taken as control, the increased amount of tumor intensity achieved by GP-FA (21-fold) was even higher than total increased amount achieved by GP (10.6-fold) plus P-FA (2.7-fold), suggesting that the enhancement of tumor accumulation by GP-FA was the result of the synergistic effect of high MW and folate conjugation. In spite of the resemblant cellular internalization of folate-modified linear polymer P-FA and non-modified P, P-FA exhibited higher tumor accumulation, in line with the lower CL of P-FA (Table 2). Taken together, based on the more pronounced EPR effect caused by high MW of graft HPMA polymers, the folate targeted system could accumulate more efficiently into tumor tissue, resulting in excellent in vivo tumor targeting of GP-FA. This phenomenon might be attributed to the lengthened circulation lifetime of graft polymers, which can drive its blood circulation, extravasation and accumulation in tumors. Alternatively, active targeting of folate attempts to enhance the retention and specificity of passive polymer delivery systems after their tumor arrival.
Most notably, the kidney accumulation was significantly higher for all polymers at 48 h compared with other organs, suggesting that the polymers could still be excreted via renal filtration.47 It should be also noted that the average fluorescence of GP and GP-FA in the liver was higher than that of P and P-FA (p < 0.05), which might be attributed to the enlarged hydrodynamic radius. Increased MW of polymer carriers may contribute to augmented capture by macrophages of the reticuloendothelial system. Nevertheless, there was still a remarkable increase in tumor accumulation achieved by GP-FA, indicating the folate decoration of graft HPMA polymer is an effective strategy for tumor targeting rather than modification of linear polymer.
Conclusion
The aim of the present study is to investigate the influence of polymer structure on the targeting efficiency of the conjugated hydrophobic ligand. Therefore, hydrophobic folates (FA) are attached to both linear HPMA polymers (P-FA, 27.3 kDa) and graft HPMA polymers (GP-FA, 117.4 kDa). Their synthesis, characterization, intracellular internalization, endocytic mechanisms, in vivo pharmacokinetics and biodistribution are studied. For P-FA, the neutrally charged feature (1.5 mV) and the comparable cell uptake with non-modified polymer P confirmed that the folate could be located inside hydrophilic polymer chain due to its hydrophobic properties. Therefore the polymer chains could hamper the interaction between the folate and its receptor. In contrast, negative zeta potential (−7.2 mV), enhanced cell uptake (2-fold higher), involvement of folate receptor mediated endocytosis and enlarged folate binding affinity were observed for GP-FA, suggesting the different exposure level of folate on the surface of P-FA and GP-FA. Furthermore, GP-FA exhibited significantly enhanced tumor accumulation relative to GP, while marginal improved tumor accumulation was observed for P-FA compared with P. These results suggested that the structure of hydrophilic polymer greatly affects the targeting efficiency of the conjugated hydrophobic ligand, and might offer some insight on the drug carrier design for tumor targeting.Acknowledgements
The research described above was supported by the National Natural Science Foundation of China (81473167) and Doctoral Fund of Ministry of Education of China (2013018111001).References
- J. Kopecek, Adv. Drug Delivery Rev., 2013, 65, 49–59 CrossRef CAS PubMed.
- J. Yang and J. Kopeček, J. Controlled Release, 2014, 190, 288–303 CrossRef CAS PubMed.
- H. Maeda, J. Controlled Release, 2012, 164, 138–144 CrossRef CAS PubMed.
- B. Daglar, E. Ozgur, M. E. Corman, L. Uzun and G. B. Demirel, RSC Adv., 2014, 4, 48639–48659 RSC.
- J. Fang, H. Nakamura and H. Maeda, Adv. Drug Delivery Rev., 2011, 63, 136–151 CrossRef CAS PubMed.
- Y. Yang, Z. Zhou, S. He, T. Fan, Y. Jin, X. Zhu, C. Chen, Z.-R. Zhang and Y. Huang, Biomaterials, 2012, 33, 2260–2271 CrossRef CAS PubMed.
- D. B. Pike and H. Ghandehari, Adv. Drug Delivery Rev., 2010, 62, 167–183 CrossRef CAS PubMed.
- X. Huang, X. Peng, Y. Wang, Y. Wang, D. M. Shin, M. A. El-Sayed and S. Nie, ACS Nano, 2010, 4, 5887–5896 CrossRef CAS PubMed.
- H. S. Qhattal, T. Hye, A. Alali and X. Liu, ACS Nano, 2014, 8, 5423–5440 CrossRef CAS PubMed.
- M. Barz, F. Canal, K. Koynov, R. Zentel and M. J. Vicent, Biomacromolecules, 2010, 11, 2274–2282 CrossRef CAS PubMed.
- F. Canal, M. J. Vicent, G. Pasut and O. Schiavon, J. Controlled Release, 2010, 146, 388–399 CrossRef CAS PubMed.
- P. M. Valencia, M. H. Hanewich-Hollatz, W. Gao, F. Karim, R. Langer, R. Karnik and O. C. Farokhzad, Biomaterials, 2011, 32, 6226–6233 CAS.
- K. M. McNeeley, A. Annapragada and R. V. Bellamkonda, Nanotechnology, 2007, 18, 385101 CrossRef.
- K. M. McNeeley, E. Karathanasis, A. V. Annapragada and R. V. Bellamkonda, Biomaterials, 2009, 30, 3986–3995 CrossRef CAS PubMed.
- K. Ulbrich, T. Etrych, P. Chytil, M. Jelınková and B. Řıíhová, J. Controlled Release, 2003, 87, 33–47 CrossRef CAS.
- T. Etrych, P. Chytil, T. Mrkvan, M. Šírová, B. Říhová and K. Ulbrich, J. Controlled Release, 2008, 132, 184–192 CrossRef CAS PubMed.
- T. Etrych, J. Strohalm, M. Sirova, B. Tomalova, P. Rossmann, B. Rihova, K. Ulbrich and M. Kovar, Polym. Chem., 2015, 6, 160–170 RSC.
- L. Li, Q.-Q. Yang, Z. Zhou, J.-J. Zhong and Y. Huang, Biomaterials, 2014, 35, 5171–5187 CrossRef CAS PubMed.
- Y.-S. Liu, H.-Y. Chen, J.-A. Yeh and L.-F. Wang, RSC Adv., 2014, 4, 59548–59557 RSC.
- R. Duncan, Adv. Drug Delivery Rev., 2009, 61, 1131–1148 CrossRef CAS PubMed.
- J. Kopeček and P. Kopečková, Adv. Drug Delivery Rev., 2010, 62, 122–149 CrossRef PubMed.
- T. Lammers, Adv. Drug Delivery Rev., 2010, 62, 203–230 CrossRef CAS PubMed.
- B. S. Tucker and B. S. Sumerlin, Polym. Chem., 2014, 5, 1566–1572 RSC.
- K. Ulbrich, V. Subr, J. Strohalm, D. Plocova, M. Jelinkova and B. Rihova, J. Controlled Release, 2000, 64, 63–79 CrossRef CAS.
- P. Rejmanová, J. Labský and J. Kopeček, Macromol. Chem. Phys., 1977, 178, 2159–2168 CrossRef.
- V. Omelyanenko, P. Kopeckova, C. Gentry and J. Kopecek, J. Controlled Release, 1998, 53, 25–37 CrossRef CAS.
- K. Ulbrich, T. Etrych, P. Chytil, M. Jelinkova and B. Rihova, J. Drug Targeting, 2004, 12, 477–489 CrossRef CAS PubMed.
- V. Šubr and K. Ulbrich, React. Funct. Polym., 2006, 66, 1525–1538 CrossRef PubMed.
- V. Subr, C. Konak, R. Laga and K. Ulbrich, Biomacromolecules, 2006, 7, 122–130 CrossRef CAS PubMed.
- T. Etrych, J. Strohalm, P. Chytil, P. Černoch, L. Starovoytova, M. Pechar and K. Ulbrich, Eur. J. Pharm. Sci., 2011, 42, 527–539 CrossRef CAS PubMed.
- G. L. Ellman, Arch. Biochem. Biophys., 1959, 82, 70–77 CrossRef CAS.
- H. Wang, P. Zhao, X. Liang, X. Gong, T. Song, R. Niu and J. Chang, Biomaterials, 2010, 31, 4129–4138 CrossRef CAS PubMed.
- T. Allen and A. Chonn, FEBS Lett., 1987, 223, 42–46 CrossRef CAS.
- J. Kopeček, P. Kopečková, T. Minko and Z.-R. Lu, Eur. J. Pharm. Biopharm., 2000, 50, 61–81 CrossRef.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., 2002, 114, 2708–2711 CrossRef.
- C. Boyer, J. Liu, V. Bulmus, T. P. Davis, C. Barner-Kowollik and M. H. Stenzel, Macromolecules, 2008, 41, 5641–5650 CrossRef CAS.
- W. Chen, L. A. Shah, L. Yuan, M. Siddiq, J. Hu and D. Yang, RSC Adv., 2015, 5, 7559–7566 RSC.
- Y. L. Li, L. Zhu, Z. Liu, R. Cheng, F. Meng, J. H. Cui, S. J. Ji and Z. Zhong, Angew. Chem., Int. Ed., 2009, 48, 9914–9918 CrossRef CAS PubMed.
- C. Zhang, D. Pan, K. Luo, N. Li, C. Guo, X. Zheng and Z. Gu, Polym. Chem., 2014, 5, 5227–5235 RSC.
- R. Zhang, K. Luo, J. Yang, M. Sima, Y. Sun, M. M. Janát-Amsbury and J. Kopeček, J. Controlled Release, 2013, 166, 66–74 CrossRef CAS PubMed.
- H. J. Greyner, T. Wiraszka, L. S. Zhang, W. M. Petroll and M. E. Mummert, Matrix Biol., 2010, 29, 503–510 CrossRef CAS PubMed.
- E. Moradi, D. Vllasaliu, M. Garnett, F. Falcone and S. Stolnik, RSC Adv., 2012, 2, 3025–3033 RSC.
- D. Yao, M. Ehrlich, Y. I. Henis and E. B. Leof, Mol. Biol. Cell, 2002, 13, 4001–4012 CrossRef CAS PubMed.
- N. Araki, M. T. Johnson and J. A. Swanson, J. Cell Biol., 1996, 135, 1249–1260 CrossRef CAS.
- S. Xiang, H. Tong, Q. Shi, J. C. Fernandes, T. Jin, K. Dai and X. Zhang, J. Controlled Release, 2012, 158, 371–378 CrossRef CAS PubMed.
- T. Lammers, F. Kiessling, W. E. Hennink and G. Storm, J. Controlled Release, 2012, 161, 175–187 CrossRef CAS PubMed.
- Z. R. Lu, Adv. Drug Delivery Rev., 2010, 62, 246–257 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra16085a |
| This journal is © The Royal Society of Chemistry 2015 |