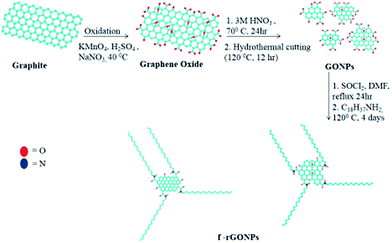
Stacking of ultra-thin reduced graphene oxide nanoparticles in supramolecular structures for optoelectronic applications†
Manish Kumar and
Sandeep Kumar*
Raman Research Institute, C. V. Raman Avenue, Sadashivanagar, Bangalore - 560 080, India. E-mail: skumar@rri.res.in; Fax: +91 80 23610492; Tel: +91 80 23610122
First published on 14th January 2015
Abstract
Octadecylamine functionalized ultra-thin reduced graphene oxide nanoparticles were synthesized and dispersed in the supramolecular order of discotic liquid crystals for the first time. The insertion and properties of the graphene nanoparticles in the columnar mesophase were studied using field emission scanning electron microscopy, atomic force microscopy, Raman spectroscopy, UV-vis spectroscopy, photoluminescence spectroscopy, polarized optical microscopy, differential scanning calorimetry, X-ray diffraction and DC conductivity. Experimental studies indicate the stacking of two-dimensional graphene nanoparticles in the supramolecular order of the columnar mesophase. The dispersion of graphene nanoparticles improves the order in the columnar phase and thus enhances the conductivity of the system.
1. Introduction
Supramolecular structures formed due to non-covalent interactions in functionalized disc-shaped molecules, commonly referred to as discotic liquid crystals (DLCs), are of enormous scientific interest because of their extraordinary unidirectional charge and energy migration properties. As these systems are of great fundamental and technological importance, significant research work is going on around the globe and has been reviewed in several articles, for example, see ref. 1–19.A majority of discotic liquid crystals are derived from polycyclic aromatic cores such as, triphenylene, anthraquinone, phthalocyanine, etc., which possess strong π–π interactions favouring columnar stacking of the molecules. In the columnar mesophase of DLCs, the aromatic cores are oriented in columns separated by molten aliphatic hydrocarbon chains. The intra-columnar (core–core) separation in a columnar mesophase is usually of the order of 0.35 nm while the inter-columnar (neighbouring columns) separation is generally in the range of 2–4 nm, depending on the length of the flexible chains. Therefore, intra-columnar interactions are much stronger than inter-columnar interactions. The columnar arrangement of aromatic cores can transport charge efficiently along the columns in quasi one dimension and, therefore, the electrical conductivity along the columns in columnar mesophases has been observed to be several orders of magnitude greater than that in perpendicular direction.20 As the charge migration is one of the most important factors in opto-electronic devices such as photovoltaic solar cells, light emitting diodes, and gas sensors, the use of DLCs in such devices could be of great importance.
The charge carrier mobility may be directly correlated to the molecular ordering in organic materials. Increasing the order in columnar structures is expected to enhance the charge carrier mobility, and the molecular order could be enhanced by enlarging the size of the polycyclic aromatic core resulting in intense π–π interactions. This concept has been well explored in the field of DLCs and an empirical relationship between charge mobility and core size has also been proposed.21 Mullen and co-workers have explored the ‘bottom-up’ approach to build DLCs derived from large polycyclic aromatic hydrocarbon (PAH) cores, defined as ‘nano-graphenes’.22–24 The dispersion of large size discotics in archetypal DLCs improves the physical properties of the system significantly due to complimentary polytopic interactions.25 Working on the ‘top-down’ approach, we have now prepared small size graphene nanoparticles to disperse in the columnar phase of DLCs.
Carbon nanomaterials such as, carbon nanotubes, fullerenes, graphene, graphene oxide, and reduced graphene oxide have attracted much attention due to their extraordinary electronic and physical properties. All these materials have been coupled with liquid crystals to hybridize their properties.26–32 Graphene nanoparticles are ultra-thin one- or few-layered pieces of graphene sheets with a diameter range of 50–70 nm bearing oxygen functional groups on their edges and basal planes. They fall in between graphene and graphene quantum dots (GQDs) and are expected to have excellent properties similar to those of bulk graphene and tiny graphene quantum dots due to their edge effects and quantum confinement.33–40
Previously, we have prepared hybrid composites of different monomeric and polymeric DLCs doped with various metallic, semiconducting and carbon NPs such as gold nanoparticles,41,42 CdSe and CdTe quantum dots,43a,43b carbon nanotubes,44,45 and graphene.26 The dispersion of these nanoparticles in DLCs does not impart much effect on their mesomorphism but improves the physical properties significantly. Here in this paper, we discussed for the first time the effects of functionalized reduced graphene oxide nanoparticle (f-rGONP) dispersion on the physical properties of triphenylene based DLCs.
2. Experimental section
2.1. Materials
Graphite powder (325 mesh), H2SO4 (conc. 80%), nitric acid (conc. 80%), sodium nitrate (NaNO3), hydrochloric acid (conc. 80%), hydrogen peroxide (H2O2), potassium permanganate (KMnO4), 30% ammonia water (NH3·H2O), and octadecylamine (ODA) were obtained from Sigma Aldrich Chemical Co. and all of them were of analytical grade.2.2. Sample characterization
Absorption and fluorescence spectra were recorded on a Perkin Elmer UV-vis-lambda 35 double beam-spectrophotometer and a Perkin Elmer Fluormax spectrophotometer, respectively. Atomic force microscope images (AFM) were taken using a Pico plus (Molecular imaging) AFM in AC tapping mode. Scanning electron microscope images were taken using a ZEISS Ultra-plus (40-98) FE-SEM. Optical textures of mesophases were observed using an Olympus-POM-018 polarized optical microscope. DSC of the liquid crystals and composites was measured in the temperature range of 5 to 150 °C using a Perkin-Elmer Pyris-1 DSC. X-Ray powder diffraction (XRD) patterns were obtained using a DY 1042-Empyrean X-ray diffractometer with a Pixel 3D detector and Cu-Kα radiation. Raman spectra were recorded on a Jobin Yuon Heriba TX64000 with an incident laser of λex = 540 nm on a flat stage.2.3. Synthesis of ultra-thin graphene oxide
Graphene oxide (GO) was prepared from natural graphite powder using the modified Hummers method.46 Pre-treated graphite powder (2 g), NaNO3 (1 g), and concentrated H2SO4 (60 mL) were placed in a round bottom-flask. KMnO4 (6 g) was added in small portions under stirring to prevent the temperature exceeding by more than 5 °C and the temperature of the mixture was kept below 20 °C using an ice-bath. After the addition of KMnO4, the ice-bath was removed and the temperature was brought to 40 °C and maintained for 30 min. After further vigorous stirring for 1 day at room temperature, the reaction was quenched by the addition of 30% H2O2 solution (25 mL) and DI water (280 mL). The mixture was filtered and washed 3 times by repeated centrifugation and filtration, first with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 M HCl
10 M HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (100 mL) solution followed by DI water (100 mL). The final product was subjected to ultra-sonication in water for 12 h.
H2O (100 mL) solution followed by DI water (100 mL). The final product was subjected to ultra-sonication in water for 12 h.
2.4. Preparation of octadecylamine functionalized reduced graphene oxide nanoparticles (f-rGONPs)
Graphene nanoparticles were prepared from ultra-thin oxidized graphene sheets following the pre-described method for the synthesis of graphene quantum dots with slight modifications.47 The doubly oxidised GO sample (0.05 g) was treated with aqueous nitric acid solution (3 M) at 70 °C for 24 h. After the reaction, the solution was cooled to room temperature and diluted with double distilled water (150 mL), and the pH was adjusted to 7 with NaOH. The resulting suspension was filtered through a 200 nm PTFE membrane. The solution was then transferred into a 50 mL Teflon-lined stainless-steel autoclave and heated at 120 °C for 12 h. The solution was further purified with a 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MWCO membrane for one week to remove metal traces and impurities. The GONPs showed strong green fluorescence.
000 MWCO membrane for one week to remove metal traces and impurities. The GONPs showed strong green fluorescence.
To functionalize the graphene oxide nanoparticles with octadecylamine, we followed the procedure of Haddon et al.50 Graphene nanoparticles (100 mg) were added to N,N-dimethylformamide (DMF) (0.5 mL) and refluxed in SOCl2 (20 mL) at 70 °C for 24 h. The excess of SOCl2 was distilled off after the reaction and the product was treated with an excess of octadecylamine (1 g) at 120 °C for 4 days at room temperature. The resultant product was washed with hot ethanol several times to remove the excess of unreacted impurities.
2.5. Preparation of composites
The two discotic liquid crystals, namely hexakis(butyloxy)triphenylene (HAT4) and hexakis(pentyloxy)triphenylene (HAT5), were synthesized as reported by us earlier.51,52 Nanocomposites having 1% of f-rGONPs in HAT4 and HAT5 were prepared by mixing the two components in chloroform. f-rGONP (1 mg) was taken in chloroform (5 mL) and sonicated for 2 h. To this solution, the triphenylene derivative (99 mg) was added and the mixture was further sonicated for 2 h to ensure homogenous composites. After that, the solvent was removed via evaporation under a N2 flow and the dried sample was used for further studies. The synthetic route is depicted in Scheme 1.3. Results and discussion
3.1. Morphology
The AFM image in Fig. 1a demonstrates the topological morphology of the f-rGONPs. The height of the f-rGONPs is between 0.8–2.5 nm, corresponding to 1–2 layers of the functionalized graphene. The average height of the f-rGONPs was calculated to be about 1.6 nm. The size distribution of the f-rGONPs is about 55–85 nm and this was further supported with Field Emission Scanning Electron Microscopy (FESEM) images which indicate an average size of 70 nm (Fig. S1a†). | ||
| Fig. 1 Morphology of graphene nanoparticles: (a) AFM image of f-rGONPs; (b) height profile of f-rGONPs. | ||
3.2. XRD and Raman spectroscopy
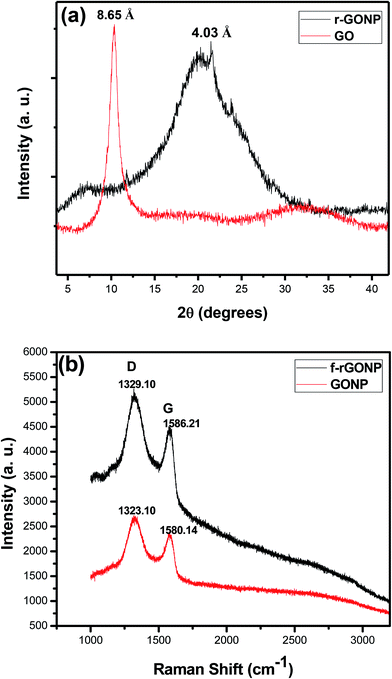
The XRD patterns of typical graphene oxide (GO) and reduced graphene oxide nanoparticles (r-GONPs) are shown in Fig. 2a. The r-GONPs have a broader (002) peak centered at around 21.5° and for GO a sharp peak is centered at around 10.30°. The interlayer spacing of the r-GONPs is 4.03 Å which is broader than that of graphite and similar to that of the GQDs.34,35 For the ODA functionalized GONPs an additional small protuberance at 2θ ≈ 21.5° can be found, likely resulting from a trace amount of the crystalline structure formed by the ordered alignment of ODA molecular chains on the GONP surfaces. Upon oxidation, the layers are buckled and the interlayer spacing is doubled to graphite due to the arrangement of the oxygen atoms in a rectangular pattern in between layers, also due to the fact that the edge of each layer is covalently connected to carboxyl and carbonyl groups (i.e. 8.65 Å). After chemical reduction with octadecylamine, for these graphene oxide nanoparticles the sharp (002) peak of graphite oxide disappeared while another broad peak at around 21.5° showed up and the interlayer spacing is about 4.03 Å. The disappearance of the sharp peak and the reduction of the interlayer spacing can be attributed to the exfoliation of the layered structures of graphite oxide. The broad peak may stem from the partial restacking of exfoliated graphene layers, removal of the oxygen-containing functional groups from the edges and between layers and the formation of turbostratically stacked graphene sheets.53Fig. 2b shows the Raman spectra of the graphene oxide nanoparticles (GONPs) and f-rGONPs. The Raman spectrum of the GONPs has a D-band at 1323.10 cm−1 and a G-band at 1580.14 cm−1 with an intensity ratio ID/IG of 1.14, and for f-rGONP the D-band and the G-band are located at 1329.10 and 1586.21 cm−1, respectively, with an intensity ratio ID/IG of 1.15, which is similar to that of GQDs.36 The G-band is assigned to the in-plane sp2 carbon–carbon bond stretching motion and the D-band is induced by disorders and edges. The intensity ratio of the two bands ID/IG is widely used to estimate the degree of disorder and the crystallite size in graphitic materials. The minor red shift in the D-band could be due to the functionalization with octadecylamine and the intensity of the D-peak is also increased, which demonstrates an abundance of new defects, edges and disorders in the graphene nanosheets, where functionalization breaks up the sp2 carbon network, and many sp3 carbon atoms have been added into the f-rGONPs suggesting partial regeneration of the electronic π-conjugation.54,55 This is probably owing to the structural distortions induced by the bulky alkyl chains of octadecylamine. The results from the XRD patterns and Raman spectra imply that the structure of the graphene nanoparticles has been maintained after chemical functionalization with octadecylamine.
3.3. Optical properties of GONPs: UV-vis and fluorescence spectroscopy
Fig. 3a shows the normalized UV-visible absorption spectrum of the GONPs. A typical weak and broader absorption peak was observed at around 302 nm which is similar to that previously reported for GQDs and assigned to the π–π* transition of the graphitic sp2 domains.47 This absorption peak also appears for the f-rGONPs at 300.78 nm (Fig. S2a†) which shows that functionalization had no effect on the absorption properties of the GONPs. A bright blue strong fluorescence peak was observed at 415 nm (Fig. 3b) when the GONPs were excited at 330 nm. The full width at half maximum (FWHM) is about 80 nm, which approximates to that reported for GQDs.37 | ||
| Fig. 3 (a) UV-vis absorption spectrum of GONPs, and (b) PL spectrum of GONPs at an excitation wavelength of 330 nm (dispersed in water). | ||
For the f-rGONPs, PL was observed at 476 nm with a FWHM of 104 nm when excited at 400 nm (Fig. S2b†). According to previous reports, this fluorescence of the GONPs is similar to that of GQDs and originated from the zigzag sites and edge structures of graphene.38 When these GONPs were excited at wavelengths from 330 to 460 nm, the PL peaks shifted from 465 to 525 nm and the intensity of the PL also decreased. This behaviour in carbon based materials was reported previously and it may be the result of the surface defects of GONPs.37,48,49
3.4. Polarized optical microscopy (POM)
The mesomorphic and thermal behaviours of nanocomposites formed by dispersing 1% f-rGONPs in hexakis(butyloxy)triphenylene (HAT4) and hexakis(pentyloxy)triphenylene (HAT5) were investigated using polarizing optical microscopy (POM) and differential scanning calorimetry (DSC) to verify the existence of the columnar mesophase of doped DLCs. Fig. 4a and b show the typical polarizing optical micrographic textures for the composites 1% f-rGONP/HAT4 and 1% f-rGONP/HAT5. They were similar to the POM textures of pure HAT4 and HAT5 indicating a homogeneous dispersion of f-rGONP in the DLCs without any phase segregation. | ||
| Fig. 4 Polarizing optical microscope images of the columnar phases of (a) 1% f-rGONP/HAT4 and (b) 1% f-rGONP/HAT5 at 120 °C under crossed polarizers (magnification 63×). | ||
3.5. FESEM
The existence of f-rGONPs in the mesophase was confirmed using FESEM. FESEM images were taken at a very low energy of 3 keV to prevent melting of the samples due to local heating. The FESEM images of HAT4 and HAT5 show rod like and layered like structures, respectively (Fig. 5a and d). The FESEM images of the 1% f-rGONP/HAT4 and 1% f-rGONP/HAT5 composites clearly show the presence of GONPs in the composites. In Fig. 5b, c, e and f we can clearly see GONPs present in the nanocomposite systems.3.6. Small angle X-ray scattering (SAXS)
The liquid crystalline phases of the nanocomposites were further studied in a SAXS study. The SAXS patterns of the composite and pure DLC were recorded at 100 °C and the intensity vs. 2θ diffraction patterns for 1% f-rGONP/HAT4 and 1% f-rGONP/HAT5 are shown in Fig. 6. In the small angle region, the diffraction peaks in both nanocomposites follow the typical Bragg diffractions of hexaalkoxytriphenylene DLC with d-spacing in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) √3
√3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, typical for a 2-dimensional columnar hexagonal phase. This confirms that the columnar hexagonal order is retained after the addition of f-rGONPs.
2, typical for a 2-dimensional columnar hexagonal phase. This confirms that the columnar hexagonal order is retained after the addition of f-rGONPs.
 | ||
| Fig. 6 Intensity vs. 2θ plots for (a) HAT4 and 1% f-rGONP/HAT4, and (b) HAT5 and 1% f-rGONP/HAT5 at 100 °C. | ||
The pure DLCs and their nanocomposites exhibit similar diffraction peaks (Fig. 6), however, a notable change can be seen in the XRD of the 1% f-rGONP/HAT5 sample. The d spacing of HAT4 is 16 Å and the core–core separation is 3.46 Å, but the composite 1% f-rGONP/HAT4 shows a d spacing of 16.04 Å with a core–core separation value of 3.43 Å. The core–core separation of the 1% f-rGONP/HAT5 composite is reduced to 3.54 Å compared to 3.61 Å in HAT5 and the d spacing is unchanged in 1% f-rGONP/HAT5, i.e. 16.86 Å. This reflects a dense packing of discotic molecules in the composite. The rGONPs were intercalated between discs and reduced the distance between two cores which leads to the formation of a better conduction pathway in the nanocomposite.
3.7. Differential scanning calorimetry (DSC)
Fig. 7a and b show the DSC plots for HAT4 and 1% f-rGONP/HAT4, and HAT5 and 1% f-rGONP/HAT5, respectively, recorded at a rate of 10 °C min−1. It is clear from the plots that the phase transitions are not significantly changed after doping with GONPs. In the 1% f-rGONP/HAT5 composite, a minor increase in the melting and isotropic temperatures was observed (Table 1). This is possibly due to the insertion of GONPs in between the aromatic cores which increases the order and compactness in the core–core separation, which was also verified in the X-ray diffraction (XRD) studies. However, in the case of the 1% f-rGONP/HAT4 composite, a minor decrease in the melting and isotropic temperatures was observed. As HAT4 possesses a columnar hexagonal plastic (Colhp) phase, which is more ordered than the Colh phase observed in HAT5, it could be possible that the order of the Colhp phase of HAT4 is slightly reduced upon GONP doping. The enthalpy of the phase transition decreased from 36.07 to 30.60 J g−1 in the mesophase and from 32.639 to 14.08 J g−1 in the isotropic phase for 1% f-rGONP/HAT4. In the 1% f-rGONP/HAT5 composite it increased from 44.97 to 45.72 J g−1 in the mesophase and from 12.68 to 12.85 J g−1 in the isotropic phase. This again indicates that the Colh phase of HAT5 stabilises upon GONP dispersion while the Colhp phase of HAT4 is destabilised upon GONP dispersion. | ||
| Fig. 7 DSC curves of (a) undoped HAT4 (black) and 1% f-rGONP/HAT4 (red), and (b) undoped HAT5 (black) and 1% f-rGONP/HAT5 (red), all are recorded at a scan rate of 10 °C min−1. | ||
| Thermal transition (°C)/enthalpy (J g−1) | ||
|---|---|---|
| Composites | Heating scan | Cooling scan |
| HAT4 | Cr 91.69(36.07) Colhp 147.19(32.63) I | I 143.21 Colhp 79.39 Cr |
| 1% f-rGONP/HAT4 | Cr 90.17(30.60) Colhp 137.54(14.08) I | I 133.20 Colhp 78.55 Cr |
| HAT5 | Cr 65.95(44.97) Colh 122.49(12.68) I | I 120.84 Colh 50.26 Cr |
| 1% f-rGONP/HAT5 | Cr 66.36(45.72) Colh 122.58(12.85) I | I 121.25 Colh 50.41 Cr |
3.8. DC conductivity
The temperature dependant DC conductivity measurements of the pure compounds and nanocomposites were carried out with unaligned samples using a four-point probe to examine the effect of graphene oxide nanoparticles on the electrical properties of the DLCs. It is observed that the DC conductivity of undoped and doped DLCs in the crystalline phase is very much lower than for the columnar hexagonal phase. Undoped HAT4 and HAT5 show conductivities around 10−9 S m−1 at 135 °C. The conductivity plots shown in Fig. 8 show the variation in the conductivity of the nanocomposites of DLCs with the temperature. The doped nanocomposites show an enhancement in the conductivity by an order of five. The conductivity plots of the 1% f-rGONP/HAT4 and 1% f-rGONP/HAT5 composites show an increase in conductivity with the temperature. It is observed that the electrical conductivity of 1% f-rGONP/HAT4 at the columnar plastic phase is 2.26 × 10−7 S m−1. The conductivity increases at the columnar hexagonal phase (mesophase) with increasing temperature from 1.56 × 10−7 S m−1 and finally reaches a value of 6.53 × 10−5 S m−1 for 1% f-rGONP/HAT4 near the isotropic phase. The conductivity of 1% f-rGONP/HAT5 in the columnar phase is 1.87 × 10−6 S m−1 and increases at the columnar hexagonal phase (mesophase) with increasing temperature from 2.77 × 10−5 S m−1 and finally reaches a value of 1.01 × 10−4 S m−1 near the isotropic phase and then decreases to 1.30 × 10−5 S m−1 in the isotropic phase. | ||
| Fig. 8 The DC conductivity values as a function of the temperature for the (a) undoped HAT4 and 1% f-rGONP/HAT4, and (b) undoped HAT5 and 1% f-rGONP/HAT5. | ||
The enhancement in the conductivity can be ascribed to the more ordered arrangement of GONPs and DLCs. The f-GONPs were inserted in between the discotic cores and assembled a bridge-like pathway for the faster conduction of π electrons from a core to a GONP and to a core. The charge carrier mobility and the transport phenomenon may be directly correlated to the molecular ordering in discotics, thus increasing the order in columnar structures is expected to enhance the charge carrier mobility through a hole hopping and electron tunnelling mechanism between the localised sites involving both the f-rGONPs and discotic aromatic cores. The changes in conductivity may be ascribed to the alignment of domains and is due to the formation of HATn+ radical cations along the columns which provides a quasi-one-dimensional path for the transport of charges, and in the isotropic phase the conductivity started to decrease due to the disruption of the columns.56 Also, limiting the molecular rotation within the columns leads to a decrease in the degrees of freedom within the mesophase, and therefore the charge carrier units are mobile only in one direction. In the crystal phase, charge carrier units are randomly arranged but upon increasing the temperature to the columnar mesophase phase (liquid crystalline phase) these charge carrier units start to be uni-directionally aligned and self-assemble with the f-rGONPs in a more ordered system, which continues to increase with increasing temperature up to the isotropic phase. The high conductivity in the isotropic phase could be due to the presence of some π-stacked domains of DLC molecules and f-rGONPs and/or the high ionic mobility at higher temperatures. These results are in accordance with previously published results on nanoparticle doped DLC systems.26,56
4. Conclusion
In conclusion, we have shown that small graphene nanoparticles of sizes in between graphene and graphene quantum dots can be easily prepared and functionalized. These nanoparticles can be efficiently dispersed in the supramolecular order of DLCs. The presence of NPs in the composites can be viewed in the FESEM images. The graphene nanoparticles are inserted in between the cores of the DLCs due to strong π–π interactions and form an ordered supramolecular structure with DLCs. The ordered supramolecular structure is achieved by forming a bridge of DLC–GONP–DLC through self-organization. The DLC–graphene nanoparticle composites exhibit a higher conductivity compared to that of the pure DLCs. Such supramolecular nanostructures could be useful for various device applications such as photovoltaic solar cells, gas sensors, one-dimensional conductors, etc.Acknowledgements
We thank Mr A. Dhason and Mrs K. N. Vasudha for their technical support in the characterization of the samples.References
- Handbook of Liquid Crystals, ed. J. Goodby, P. J. Collings, T. Kato, C. Tschierske, H. Gleeson and P. Raynes, Wiley-VCH, Weinheim, 2014, vol. 4, part III, p. 467 Search PubMed.
- S. Kumar, Isr. J. Chem., 2012, 52, 820 CrossRef CAS.
- S. Kumar, Chemistry of Discotic Liquid Crystals: From Monomers to Polymers, CRC Press, Boca Raton, FL, 2011 Search PubMed.
- H. K. Bisoyi and S. Kumar, Liq. Cryst., 2011, 38, 1427 CrossRef CAS.
- R. J. Bushby and K. Kawata, Liq. Cryst., 2011, 38, 1415 CrossRef CAS.
- W. Pisula, X. Feng and K. Mullen, Chem. Mater., 2011, 23, 554 CrossRef CAS.
- H. K. Bisoyi and S. Kumar, Chem. Soc. Rev., 2010, 39, 264 RSC.
- S. Kumar, Liq. Cryst., 2009, 36, 607 CrossRef CAS.
- J. W. Goodby, I. M. Saez, S. J. Cowling, V. Gortz, M. Draper, A. W. Hall, S. Sia, G. Cosquer, S. E. Lee and E. P. Raynes, Angew. Chem., Int. Ed., 2008, 47, 2754 CrossRef CAS PubMed.
- C. Tschierske, Chem. Soc. Rev., 2007, 36, 1930 RSC.
- S. Sergeyev, W. Pisula and Y. H. Geerts, Chem. Soc. Rev., 2007, 36, 1902 RSC.
- J. Wu, W. Pisula and K. Mullen, Chem. Rev., 2007, 107, 718 CrossRef CAS PubMed.
- S. Laschat, A. Baro, N. Steinke, F. Giesselmann, C. Hagele, G. Scalia, R. Judele, E. Kapatsina, S. Sauer, A. Schreivogel and M. Tosoni, Angew. Chem., Int. Ed., 2007, 46, 4832 CrossRef CAS PubMed.
- T. Kato, N. Mizoshita and K. Kishimoto, Angew. Chem., Int. Ed., 2006, 45, 38 CrossRef CAS PubMed.
- S. Kumar, Chem. Soc. Rev., 2006, 35, 83 RSC.
- B. Donnio, D. Guillon, R. Deschenaux and D. W. Bruce, Comprehensive Coordination Chemistry II, ed. J. A. McCleverty and T. J. Meyer, Elsevier, Oxford, 2003, vol. 7, p. 357 Search PubMed.
- K. Ohta, K. Hatsusaka, M. Sugibayashi, M. Ariyoshi, K. Ban, F. Maeda, R. Naito, K. Nishizawa, A. M. Van de Craats and J. M. Warman, Mol. Cryst. Liq. Cryst., 2003, 397, 25 CrossRef.
- R. J. Bushby and O. R. Lozman, Curr. Opin. Solid State Mater. Sci., 2002, 6, 569 CrossRef CAS.
- V. Percec, M. Glodde, T. K. Bera, Y. Miura, I. Shiyanovskaya, K. D. Singer, V. S. K. Balagurusamy, P. A. Heiney, I. Schnell, A. Rapp, H. W. Spiess, S. D. Hudson and H. Duan, Nature, 2002, 419, 384 CrossRef CAS PubMed.
- V. S. K. Balagurusamy, S. Krishnaprasad, S. Chandrasekhar, S. Kumar, M. Manickam and C. V. Yelamaggad, Pramana, 1999, 53, 3 CrossRef CAS.
- A. M. Van de Craats and J. M. Warman, Adv. Mater., 2001, 13, 130 CrossRef CAS.
- W. Pisula, X. Feng and K. Mullen, Chem. Mater., 2011, 23, 554 CrossRef CAS.
- J. S. Wu, W. Pisula and K. Mullen, Chem. Rev., 2007, 107, 718 CrossRef CAS PubMed.
- S. Yang, R. E. Bachman, X. Feng and K. Müllen, Acc. Chem. Res., 2013, 46, 116 CrossRef CAS PubMed.
- E. O. Arikainen, N. Boden, R. J. Bushby, O. R. Lozman, J. G. Vinter and A. Wood, Angew. Chem., Int. Ed., 2000, 39(13), 2333 CrossRef CAS.
- A. B. Shivanandareddy, S. Krishnamurthy, V. Lakshminarayanan and S. Kumar, Chem. Commun., 2014, 50, 710 RSC.
- H. K. Bisoyi and S. Kumar, Chem. Soc. Rev., 2011, 40, 306 RSC.
- O. Stamatoiu, J. Mirzaei, X. Feng and T. Hegmann, Top. Curr. Chem., 2012, 318, 331 CrossRef CAS.
- Q. Li, Nanoscience with liquid crystals, Springer, 2014 Search PubMed.
- J. P. F. Lagerwall and G. Scalia, Curr. Appl. Phys., 2012, 12, 1387 CrossRef PubMed.
- S. Kumar, NPG Asia Mater., 2014, 6, e82 CrossRef CAS.
- S. Kumar, Liq. Cryst., 2014, 41, 353 CrossRef CAS.
- A. Nieto, D. Lahiri and A. Agarwal, Carbon, 2012, 50, 4068 CrossRef CAS PubMed.
- W. Choi, I. Lahiri, R. Seelaboyina and Y. S. Kang, Crit. Rev. Solid State Mater. Sci., 2010, 35, 52 CrossRef CAS.
- D. A. Dikin, S. Stankovich, E. J. Zimney, R. D. Piner, G. H. B. Dommett, G. Evmenenko, S. T. Nguyen and R. S. Ruoff, Nat. Lett., 2007, 448, 457 CrossRef CAS PubMed.
- I. K. Moon, J. Lee, R. S. Ruoff and H. Lee, Nat. Commun., 2010, 73, 1 CrossRef PubMed.
- S. Zhu, J. Zhang, C. Qiao, S. Tang, Y. Li, W. Yuan, B. Li, L. Tian, F. Liu, R. Hu, H. Gao, H. Wei, H. Zhang, H. Sun and B. Yang, Chem. Commun., 2011, 47, 6858 RSC.
- L. S. Li and X. Yan, J. Phys. Chem. Lett., 2010, 1, 2572 CrossRef CAS.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS PubMed.
- B. Partoens and F. M. Peeters, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 1 CrossRef.
- S. Kumar and V. Lakshminarayanan, Chem. Commun., 2004, 1600 RSC.
- S. Kumar, S. Pal, P. S. Kumar and V. Lakshminarayanan, Soft Matter, 2007, 3, 896 RSC.
- (a) S. Kumar and L. Sagar, Chem. Commun., 2011, 47, 12182 RSC; (b) M. Kumar and S. Kumar, RSC Adv., 2015, 5, 1262–1267 RSC.
- S. Kumar and H. K. Bisoyi, Angew. Chem., Int. Ed., 2007, 46, 1501 CrossRef CAS PubMed.
- H. K. Bisoyi and S. Kumar, J. Mater. Chem., 2008, 18, 3032 RSC.
- W. S. Hummers and R. E. Offman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- J. Shen, Y. Zhu, X. Yang and C. Li, New J. Chem., 2012, 36, 97 RSC.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726 CrossRef CAS PubMed.
- V. N. Mochalin and Y. Gogotsi, J. Am. Chem.Soc., 2009, 131, 4594 CrossRef CAS PubMed.
- S. Niyogi, E. Bekyarova, M. Itkis, J. McWilliams, M. Hamon and R. Haddon, J. Am. Chem. Soc., 2006, 128, 7720 CrossRef CAS PubMed.
- S. Kumar and M. Manickam, Chem. Commun., 1997, 1615 RSC.
- S. Kumar, Liq. Cryst., 2004, 31, 1037 CrossRef CAS.
- K. Zhang, Y. Zhang and S. Wang, Sci. Rep., 2013, 3, 3448 Search PubMed.
- J. Dong, Z. Yao, T. Yang, L. Jiang and C. Shen, Sci. Rep., 2013, 3, 1733 Search PubMed.
- X. Tian, S. Sarkar, A. Pekker, M. L. Moser, I. Kalinina, E. Bekyarova, M. E. Itkis and R. C. Haddon, Carbon, 2014, 72, 82 CrossRef CAS PubMed.
- C. Kavitha, B. S. Avinash, S. Kumar and V. Lakshminarayanan, Mater. Chem. Phys., 2012, 133, 635 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra15705b |
| This journal is © The Royal Society of Chemistry 2015 |