Fluorescent/laser dual-channel ATP sensors based on flavins†
Yu Liu,
Peng Li,
Hongwei Ma,
Ming Zhang* and
Feng Li*
State Key Lab of Supramolecular Structure and Materials, Jilin University, 2699 Qianjin Avenue, Changchun 130012, People’s Republic of China. E-mail: lifeng01@jlu.edu.cn; zhming@jlu.edu.cn
First published on 9th January 2015
Abstract
The fluorescence biomolecules flavin mononucleotide (FMN) and lumiflavin were demonstrated as ATP sensors for the first time. FMN and lumiflavin are totally compatible with the human body and can be easily obtained at low cost. The laser and fluorescence of FMN and lumiflavin in aqueous solution can efficiently discriminate ATP from other common anion such as Cl−, Br−, I−, SO42−, NO3− and PPi.
Adenosine-5′-triphosphate (ATP) serves as a phosphate donor in kinase-catalyzed protein phosphorylation and the extracellular ATP released from the cell membrane mediates many cell-to-cell signals in a wide range of physiological and pathological conditions.1 Therefore, the determination of ATP is essential to biochemical studies as well as clinical diagnosis. There have been considerable efforts to develop fluorescent or colorimetric sensors for ATP.2–21 For example, Hamachi et al. developed a series of binuclear zinc complexes as fluorescent chemosensors for ATP.2–5 Yoon et al. reported a pyrene derivative that can display a unique ratiometric fluorescence change only with ATP binding.6,7 Mohr et al. synthesized silica nanoparticles for ATP detection.9 Hong et al. presented an off–on switching fluorescent sensor showing a moderate selectivity for ATP.11 Bencini et al. developed a phenanthroline-containing polyammonium receptor that can discriminate ATP from other nucleosides through a quantitative quenching of its fluorescence emission.12,13 Morii et al. reported covalently linked fluorescent ribonucleopeptide (RNP) sensors that are capable of detecting ATP and GTP simultaneously.14 However, the above achievements still need a chemical or biological synthesis process involving two or more steps. Water solubility and biocompatibility are urgently needed for practical applications.
Flavin mononucleotide (FMN), a kind of fluorescent biomolecule produced from vitamin B2, serves as a coenzyme in a series of oxidation–reduction catalysts and is found in many types of human tissue, including heart, liver, and kidney tissue, and thus is totally compatible with the human body and can be easily obtained at low cost. FMN also has a relatively high fluorescence quantum yield of 0.23,15,16 which is superior to many other fluorescent biomolecules in the human body whose fluorescence quantum yields are very low. Nizamoglu and Yun17 demonstrated FMN to be an efficient laser gain material, and the laser based on it has lasing thresholds as low as tens of nanojoules. The merits of FMN mentioned above indicate that it should be a good fluorescent biosensor, but there has been no report about this until now.
In this work, for the first time we used the laser and fluorescence of FMN to detect ATP, which has the following advantages: (I) FMN is totally compatible with the human body and can be easily obtained at low cost; (II) the laser of FMN can efficiently discriminate ATP from other common anion such as Cl−, Br−, I−, SO42−, NO3− and PPi; (III) it exhibits fluorescence enhancement which is more desirable in sensing than fluorescence quenching, and the limits of detection (LODs) of FMN are 1.0 μM and 7.3 μM for laser and fluorescence, respectively. Moreover, the feasibility for determining ATP in urine samples was also studied, and satisfactory results were obtained. Lumiflavin has the same isoalloxazine core as FMN but lacks the side chain and it has similar properties to FMN. We performed parallel experiments of ATP detection using the laser and fluorescence of lumiflavin in aqueous solution, and obtained similar results to FMN (please see the ESI).†
We used two concave lenses (interspacing: L = 12.7 mm; curvature: 50 mm) and a 400 uL quartz container to construct a simple low-loss optical resonator. Both mirrors had a dichroic coating with high reflectivity (R > 99%) in a wavelength range of 500–560 nm and high transmission at λ < 450 nm. The quartz container was completely filled with a 100 μM aqueous solution of FMN or lumiflavin and was fixed between the two mirrors. The concave surfaces of the mirrors were used as internal reflection cavities. A Nd:YAG laser (λ = 355 nm, pulse width: 5 ns) was used to optically pump the resonator. The pump energy was adjusted using a set of neutral density filters. The output-light of the cavity was collected through a fibre-optics probe connected to a computer. The details of the materials and the measurement methods can be found in the ESI.†
Fig. 1 shows the absorption, photoluminescence (PL) and laser spectra of FMN. As can be seen, the absorption bands peaking at around 446 nm and 375 nm correspond to the transitions of the isoalloxazine chromophore from S0 to S1 and from S0 to S2, respectively.15,18 The PL spectrum of FMN is characterized by a broad structureless band peaking at around 522 nm, corresponding to the transition from S1 to S0.15 Under the irradiation of a Nd:YAG nanosecond pulse laser (355 nm), the aqueous FMN solution in the optical resonator emits a laser peaking at 567 nm. The details of the FMN laser properties can be found in the ESI.†
Upon the incremental addition of ATP, the intensities of the fluorescence and laser spectra increase and are enhanced by 3.4 and 5.5 fold until the amounts of ATP reach 180 μM and 133 μM, respectively. By linearly fitting the changes in the fluorescence and the laser (Fig. 2) as a function of the concentration of ATP, we obtain a slope of 2.9 × 107 for the laser and 4.2 × 106 for the fluorescence. According to LOD = 3SB/m,19 where SB is the standard deviation of the blank measurements and m is the slope of intensity versus sample concentration, the LODs of the laser and fluorescence sensors are calculated to be 1.0 μM and 7.3 μM, both of which are much lower than the ATP levels in the human body (4 mM in resting muscle; 2 mM in erythrocytes).20 The LOD of the laser sensor is lower by about one order of magnitude than that of the fluorescence sensor. This is because the laser has the function of amplifying the test signal.
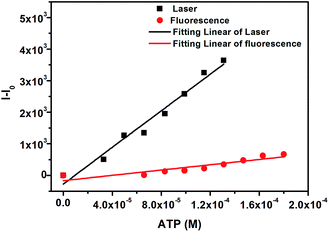 | ||
| Fig. 2 Changes (I–I0) in the fluorescence (red circle) and laser (black square) spectra of FMN (100 μM) as a function of the concentration of ATP sodium salt in aqueous solution. | ||
The selectivities of the laser and fluorescence sensors of FMN were tested against several common anions. The results are represented in Fig. 3. As can be seen, inorganic anions cannot provide significant interactions with FMN. The change ratios caused by inorganic anions are less than 21% for the laser sensor and 11% for the fluorescence sensor. On the other hand, ATP, ADP and AMP can interact well with FMN and enhance the laser and fluorescence intensities to different extents. The largest enhancement is observed in the case of ATP (308% for laser, 59% for fluorescence), followed by ADP (131% for laser, 27% for fluorescence) and AMP (45% for laser, 19% for fluorescence). The results indicate that the selectivities of the laser and fluorescence sensors are not satisfactory for discriminating ATP, ADP and AMP, but the laser and fluorescence sensors can efficiently discriminate ATP, ADP or AMP from other common anions, and thus can be used as rough sensors due to the low cost of FMN.
A study was also carried out regarding the influence of pH on the enhancement of the laser and fluorescence of FMN with ATP. For this purpose, the changes in laser and fluorescence intensity were measured in the presence of ATP (99 μM) in sodium acetate buffer solution with the pH ranging from 4.0 to 6.0, potassium dihydrogen phosphate and sodium hydroxide buffer solutions with the pH ranging from 6.0 to 7.0, and Tris–HCl buffer solution with the pH ranging from 7.0 to 8.0, as shown in the inset of Fig. 3. The results indicated that the pH value has little effect on the laser and fluorescence sensors of FMN over a wide range from 4.0 to 8.0.
According to the fact that the ability of ATP, ADP and AMP to enhance the laser and fluorescence of FMN becomes weaker with the decrease in the number of phosphates, we suppose that the hydrogen bonds between the isoalloxazine of FMN and the phosphate groups of ATP, ADP and AMP induce the enhancement of the laser and fluorescence of FMN. The absorption bands of the FMN + ATP solution have a small red-shift compared to those of the FMN solution, as shown in Fig. 1, suggesting the existence of hydrogen bonds.21,15 1H NMR spectra were also employed to clarify the interaction between ATP and FMN. Upfield shifts are observed in Fig. 4 for the aromatic protons (H1, H2 and H9) of isoalloxazine and the purine protons (H10 and H11) of ATP after mixing the ATP and FMN solutions. The upfield shifts denote an increase in the electron cloud density of the microenvironment, indicating a strong interaction between ATP and FMN.
 | ||
| Fig. 4 Partial 500 MHz 1H NMR spectra for (a) ATP (10 mM), (b) ATP (10 mM) + FMN (100 μM), and (c) FMN (100 μM) in D2O solution. | ||
In order to demonstrate the feasibility of the FMN sensor in real samples, urine samples from 5 healthy volunteers were used. Fig. 5 shows the changes in the fluorescence and laser spectra of FMN upon incremental addition of one urine sample. As can be seen, just 25 μL urine can cause obvious changes in the intensity of the fluorescence and laser. The change ratios of the intensity of the fluorescence and laser can reach 198% and 557%, respectively, after adding 175 μL urine. Thus, the fluorescence and laser of FMN in aqueous solution can be used to detect ATP in urine. It should be noted that the change ratios of the intensity of the fluorescence and laser caused by the urine samples from different volunteers are different, indicating the varying content of ATP in different urine samples.
 | ||
| Fig. 5 The fluorescence (a) and laser (b) spectra of FMN (100 μM) in aqueous solution upon incremental addition of one urine sample. | ||
We also performed parallel experiments of ATP detection using the laser and fluorescence of lumiflavin in aqueous solution. Similar results to FMN were obtained (please see ESI).†
Conclusions
In summary, we demonstrated that FMN and lumiflavin can be used as fluorescent/laser dual-channel ATP sensors in 100% aqueous solution. FMN and lumiflavin are totally compatible with and non-toxic to the human body and can be easily obtained at low cost. The LOD of the FMN laser was found to be much lower than the ATP levels in the human body (4 mM in resting muscle; 2 mM in erythrocytes). The feasibility for determining ATP in urine samples was also proven. ATP sensors from the aqueous solutions of flavins offer the possibility to roughly detect ATP at low cost in the future.Acknowledgements
This work was supported by NSFC (grant no. 21374037, 21221063 and 91233113).Notes and references
- A. V. Gourine, E. Llaudet, N. Dale and K. M. Spyer, Nature, 2005, 7047, 108 CrossRef PubMed.
- A. Ojida, H. Nonaka, Y. Miyahara, S. I. Tamaru, K. Sada and I. Hamachi, Angew. Chem., Int. Ed., 2006, 33, 5518 CrossRef PubMed.
- A. Ojida, I. Takashima, T. Kohira, H. Nonaka and I. Hamachi, J. Am. Chem. Soc., 2008, 36, 12095 CrossRef PubMed.
- T. Sakamoto, A. Ojida and I. Hamachi, Chem. Commun., 2009, 2, 141 RSC.
- Y. Kurishita, T. Kohira, A. Ojida and I. Hamachi, J. Am. Chem. Soc., 2010, 38, 13290 CrossRef PubMed.
- Z. C. Xu, K. S. Kim and J. Y. Yoon, J. Am. Chem. Soc., 2009, 131, 15528 CrossRef CAS PubMed.
- Z. Xu, D. R. Spring and J. Yoon, Chem.–Asian J., 2011, 8, 2113 Search PubMed.
- Y. Zhou, Z. C. Xu and J. Y. Yoon, Chem. Soc. Rev., 2010, 40, 2222 RSC.
- A. J. Moro, J. Schmidt and G. J. Mohr, Chem. Commun., 2011, 47, 6066 RSC.
- D. L. Lee, S. Y. Kim and J. I. Hong, Angew. Chem., Int. Ed., 2004, 43, 777 Search PubMed.
- D. L. Lee, S. Y. Kim and J. I. Hong, Tetrahedron Lett., 2007, 48, 4477 CrossRef CAS PubMed.
- C. Bazzicalupi and A. Bencini, Chem. Commun., 2006, 39, 4087 RSC.
- C. Bazzicalupi and A. Bencini, Chem. Commun., 2005, 20, 2630 RSC.
- S. Nakano, M. Fukuda, T. Tamura, R. Sakaguchi, E. Nakata and T. Morii, J. Am. Chem. Soc., 2013, 135, 3456 Search PubMed.
- P. F. Heelis, Chem. Soc. Rev., 1982, 11, 15 RSC.
- W. Holzer, J. Shirdel and P. Zirak, Chem. Phys., 2005, 308, 69 CrossRef CAS PubMed.
- S. Nizamoglu and S. H. Yun, Adv. Mater., 2013, 41, 5943 CrossRef PubMed.
- E. Sikorska, I. V. Khmelinskii and M. Prukala, J. Phys. Chem. A, 2004, 108, 1501 CrossRef CAS.
- International Union of Pure and Applied Chemistry, Pure and Applied Chemistry, 1976, vol. 45, p. 99 Search PubMed.
- A. Ghosh, P. Ronner and E. Cheong, J. Biol. Chem., 1991, 266, 22887 CAS.
- K. Yagi, N. Ohishi and K. Nishimoto, Biochemistry, 1980, 19, 1553 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra16022c |
| This journal is © The Royal Society of Chemistry 2015 |


