DOI:
10.1039/C4RA16004E
(Paper)
RSC Adv., 2015,
5, 19666-19674
Thermal and mechanical properties of azomethine functionalized cyanate ester/epoxy blends
Received
9th December 2014
, Accepted 30th January 2015
First published on 2nd February 2015
Abstract
A series of azomethine functionalized diols were synthesized by the condensation reaction of aromatic diamines with 4-hydroxy benzaldehyde. Aromatic diamines with different alkyl chain lengths have been used to prepare the bisphenols. These bisphenols were converted to their corresponding cyanate esters by treating with cyanogen bromide (CNBr) in the presence of triethyl amine (Et3N). The curing temperature was measured using differential scanning calorimetry (DSC). The maximum curing temperatures of these cyanate esters are in the range of 203–242 °C. Thermal properties of the cured cyanate esters were studied by using thermogravimetric analysis (TGA). The polymers showed excellent thermal stability (T5% was found to be in the range of 358 to 465 °C and T10% was found to be in the range of 508 to 525 °C) and the percentage of char yield at 800 °C was found to be in the range of 37.06 to 57.26. The flame retardancy of the cyanate ester resins was evaluated using the limiting oxygen index value which is in the range of 32.32 to 40.40 at 800 °C. Cyanate ester/epoxy blends were prepared and the morphology of the blends were studied by scanning electron microscopy. The mechanical properties and glass transition temperature of cyanate/epoxy ester blends was studied using dynamic mechanical analysis (DMA) and the Tg was found to increase with increasing cyanate ester content (1 to 5%).
1. Introduction
Cyanate esters are one type of thermosetting resins. On heating or irradiation either in the presence or absence of catalyst, three units of cyanate ester monomer undergo cyclotrimerization to form a symmetric triazine ring with nitrogen and carbon atoms, where the carbon atoms are linked to an ether substituent.1 The heat resistant character of cured cyanate esters (CE) make them more appealing for use in high thermal applications.2 The polycyanurates exhibit low dielectric constants and low dissipation factors. This character is well suited for making printed circuit boards in electronic industries.3–5 Radar transparency, low moisture absorption,6 very good adhesive character towards metal and high mechanical strength7–10 make cyanate esters more reliable for high-end applications in aerospace industries. However, the cured CE is inherently brittle in nature and easily undergoes microcracking under service loads. The fracture toughness of cyanate esters can be improved by blending them with thermoplastic tougheners, rubber,11 organic and inorganic fillers,6,12 POSS13–15 and thermosetting resins such as epoxy,16–20 imides21–24 and benzoxazines.25,26
The commercially available cyanate ester bisphenol A dicyanate ester (BADCY) is widely used in various fields, but further modification is required to enhance its performance properties and reduce the cost of production. The thermooxidative stability and moisture resistance were improved by introducing silicon atoms into BADCY.6 On the addition of epoxy resin into the BADCY resin, the mechanical and hot–wet properties were improved with concentration ranges of 5 to 30 wt%.20 Epoxy resins also come under thermosetting resins category and they are widely used for making advanced composites because of their excellent adhesive nature towards metals, chemical resistance and outstanding mechanical properties27 with superior dimensional stability. They undergo microcracking, which makes them unfit for purpose in high toughness applications (in engineering industries). Another disadvantage of epoxy resins is their moisture absorption.
Azomethine functionalized cyanate esters with shorter alkyl chains were prepared previously.28,29 In this study, dicyanate esters with azomethine linkages and different alkyl chains with ether linkages were prepared and the effect of the alkyl chain length between two aromatic groups on the thermal stability has been studied. The prepared cyanate esters were blended with epoxy resin in three different weight percentages. Thermal and thermomechanical properties of the CE/epoxy blends were investigated by using thermogravimetric analysis (TGA) and dynamic mechanical analysis (DMA). The morphology of the system was studied by scanning electron microscopy (SEM) techniques.
2. Experimental procedure
2.1. Materials
4-Acetamidophenol was purchased from Aldrich, USA. Acetone, 1,4-dibromo butane, 1,5-dibromo pentane, 1,6-dibromo hexane, potassium carbonate (K2CO3), cyanogen bromide (CNBr) and 4-hydroxy benzaldehyde were purchased from Sisco Research Laboratories (SRL-India). The diglycidyl ether of bisphenol-A (DGEBA) was purchased from Huntsman (India). Diaminodiphenylmethane (DDM) was purchased from Acros Organics (India). Ethanol and triethylamine (Et3N) were purchased from Merck (India). All other reagents were used as received. Triethylamine and acetone were distilled prior to use.
2.2. Measurements
Fourier transform infrared (FT-IR) spectra were recorded in an ABB Bomem MB series FT-IR spectrometer using a KBr pellet. 1H and 13C nuclear magnetic resonance measurements were carried out with a Bruker AV III spectrometer (500 MHz). Samples were prepared in CDCl3 or DMSO-d6 solutions. Melting temperatures were determined with a melting point apparatus, IA 6304, at a heating rate of 4 °C min−1. Differential scanning calorimetric (DSC) analysis for curing studies was carried out using Perkin Elmer DSC 7 model at a heating rate of 5 °C min−1 under an air atmosphere using aluminum pans. Thermogravimetric analysis (TGA) was performed using a Perkin Elmer Diamond series model at a heating rate of 10 °C min−1 under flowing air in the range of 25–800 °C.
2.3. Synthesis of 1,4-bis(4-acetamidophenoxy)butane
4-Acetamidophenol (5.0 g, 0.0330 mol) and 10.26 g of potassium carbonate (0.0742 mol) in 100 mL of acetone were taken in a 250 mL round bottomed flask. To this, 1,4-dibromobutane (3.5708 g, 0.0165 mol) in 50 mL of acetone was added drop wise. The reaction was initiated by adding trace amounts of potassium iodide. Then it was refluxed at 80 °C overnight. The mixture was cooled and poured into water (1000 mL). The white precipitate formed was separated and recrystallized from ethanol.30 Yield 89%, m.p.: 190 °C. IR (KBr, cm−1): 3336 (CONH), 1053 (–C–O–C–), 1675 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 824 (p-substituted aromatic).
O), 824 (p-substituted aromatic).
2.4. Synthesis of 1,4-bis(4-aminophenoxy)butane
1,4-Bis(4-acetamidophenoxy)butane (4.0 g, 0.0112 mol) in 100 mL ethanol was taken in a 250 mL round bottomed flask. To this, 10.0 g of sodium hydroxide (0.25 mol) dissolved in water (35 mL) was added drop wise. After the addition was completed, the mixture was refluxed at 80 °C for 8 h, cooled and poured into crushed ice. The resulting pale brown precipitate was filtered, washed with distilled water twice and recrystallized from ethanol. Yield 93%, m.p.: 140 °C. FT-IR (KBr, cm−1): 3395, 3371 (aromatic NH2), 2945 (OCH2), 835 (p-substituted aromatic). 1H-NMR (500 MHz, CDCl3, ppm): 3.7 (s, 4H, a (Ar-NH2)), 6.6 (d, 4H, b), 7.2 (d, 4H, c), 3.8 (t, 4H, d (OCH2CH2)), 1.8 (m, 4H, e (OCH2CH2)). 13C-NMR (CDCl3, ppm): C1-139.9, C2-115.7, C3-115.2, C4-149.3, C5-67.9, C6-28.8. The other two diamines were prepared in a similar way.
2.4.1. Synthesis of 1,4-bis[4(4-hydroxyphenylazomethyl)phenoxy]butane. 1,4-Bis(4-aminophenoxy)butane (2.750 g, 0.01 mol) was taken in a 250 mL round bottomed flask with 100 mL of ethanol and refluxed until it was completely dissolved in ethanol. To this 2.4662 g of 4-hydroxy benzaldehyde (0.0201 mol) in 50 mL of ethanol was added drop wise with constant stirring. The reaction was initiated by adding trace amounts of acetic acid. The mixture was refluxed for a further period of 3 h. The mixture was then cooled and poured into cold water (1.0 L). The resulting yellow precipitate was collected and recrystallized from ethanol. Yield: 91%, m.p.: 83 °C. FT-IR (KBr, cm−1): 3380 (OH), 2943 (OCH2), 1680 (HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 834 (p-substituted aromatic). 1H-NMR (DMSO-d6, ppm): 9.4 (s, 2H, a), 6.9 (d, 4H, b), 7.7 (d, 4H, c), 8.7 (s, 2H, d), 7.1 (d, 4H, e), 6.9 (d, 4H, f), 4.1 (t, 4H, g), 1.9 (m, 4H, h). 13C-NMR (500 MHz, DMSO-d6, ppm): C1-161.1, C2-116.8, C3-130.9, C4-130.1, C5-159.3, C6-145.9, C7-122.3, C8-116.8, C9-155.8, C10-66.3, C11-27.8.
N), 834 (p-substituted aromatic). 1H-NMR (DMSO-d6, ppm): 9.4 (s, 2H, a), 6.9 (d, 4H, b), 7.7 (d, 4H, c), 8.7 (s, 2H, d), 7.1 (d, 4H, e), 6.9 (d, 4H, f), 4.1 (t, 4H, g), 1.9 (m, 4H, h). 13C-NMR (500 MHz, DMSO-d6, ppm): C1-161.1, C2-116.8, C3-130.9, C4-130.1, C5-159.3, C6-145.9, C7-122.3, C8-116.8, C9-155.8, C10-66.3, C11-27.8.
2.4.2. 1,5-Bis[4(4-hydroxyphenylazomethyl)phenoxy]pentane. Yield: 83%, m.p.: 90 °C. FT-IR (KBr, cm−1): 3414 (OH), 2945 (OCH2), 1688 (HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 836 (p-substituted aromatic). 1H-NMR (DMSO-d6, ppm): 9.2 (s, 2H, a), 6.8 (d, 4H, b), 7.8 (d, 4H, c), 8.6 (s, 2H, d), 7.2 (d, 4H, e), 7.0 (d, 4H, f), 4.0 (m, 4H, g), 1.8 (m, 4H, h), 1.8 (m, 2H, i). 13C-NMR (500 MHz, DMSO-d6, ppm): C1-160.5, C2-115.8, C3-131.2, C4-129.1, C5-159.9, C6-145.5, C7-122.5, C8-115.7, C9-158.5, C10-67.3, C11-29.8, C12-22.8.
N), 836 (p-substituted aromatic). 1H-NMR (DMSO-d6, ppm): 9.2 (s, 2H, a), 6.8 (d, 4H, b), 7.8 (d, 4H, c), 8.6 (s, 2H, d), 7.2 (d, 4H, e), 7.0 (d, 4H, f), 4.0 (m, 4H, g), 1.8 (m, 4H, h), 1.8 (m, 2H, i). 13C-NMR (500 MHz, DMSO-d6, ppm): C1-160.5, C2-115.8, C3-131.2, C4-129.1, C5-159.9, C6-145.5, C7-122.5, C8-115.7, C9-158.5, C10-67.3, C11-29.8, C12-22.8.
2.4.3. 1,6-Bis[4(4-hydroxyphenylazomethyl)phenoxy]hexane. Yield: 91%, m.p.: 109 °C. FT-IR (KBr, cm−1): 3342 (OH), 2932 (OCH2), 1676 (HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) 839 (p-substituted aromatic). 1H-NMR (DMSO-d6, ppm): 9.2 (s, 2H, a), 6.9 (d, 4H, b), 7.9 (d, 4H, c), 8.6 (s, 2H, d), 7.2 (d, 4H, e), 6.9 (d, 4H, f), 4.1 (t, 4H, g), 1.7 (m, 4H, h), 1.5 (m, 2H, i). 13C-NMR (500 MHz, DMSO-d6, ppm): C1-160.8, C2-116.1, C3-131.1, C4-128.9, C5-160.1, C6-144.8, C7-121.5, C8-115.9, C9-157.8, C10-68.9, C11-29.6, C12-25.8.
N) 839 (p-substituted aromatic). 1H-NMR (DMSO-d6, ppm): 9.2 (s, 2H, a), 6.9 (d, 4H, b), 7.9 (d, 4H, c), 8.6 (s, 2H, d), 7.2 (d, 4H, e), 6.9 (d, 4H, f), 4.1 (t, 4H, g), 1.7 (m, 4H, h), 1.5 (m, 2H, i). 13C-NMR (500 MHz, DMSO-d6, ppm): C1-160.8, C2-116.1, C3-131.1, C4-128.9, C5-160.1, C6-144.8, C7-121.5, C8-115.9, C9-157.8, C10-68.9, C11-29.6, C12-25.8.
2.5. Synthesis of cyanate ester monomers
A 250 mL three-necked round bottomed flask, equipped with a magnetic stirring device and a nitrogen inlet, was charged with 3.5 g of the bisphenol (1,4-bis[4(4-hydroxyphenylazomethyl)phenoxy]butane) (0.0114 mol). The flask was maintained at −15 °C and then a solution of 2.3 g (0.0228 mol) of CNBr in acetone (50 mL) was added with constant stirring. To this mixture, 3.1 mL (0.0228 mol) of triethyl amine was added drop wise with continuous stirring. After the complete addition of triethyl amine, the reaction mixture was stirred for a further period of 2 h while maintaining the bath temperature at −15 °C. The temperature was then raised to room temperature. The mixture was poured into cold distilled water to precipitate the cyanate ester. A light brown product (yield: 91%) was obtained. All other prepared bisphenols were converted into their corresponding dicyanate ester monomers in a similar way.
2.5.1. 1,4-Bis[4(4-cyanatophenylazomethyl)phenoxy]butane (CE1). Yield: 91%, m.p.: 91 °C. FT-IR (KBr, cm−1): 2198, 2212 (OCN), 2945 (OCH2), 1681 (HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 834 (p-substituted aromatic). 1H-NMR (DMSO-d6, ppm): 6.9 (d, 4H, a), 7.6 (d, 4H, b), 8.6 (s, 2H, c), 7.1 (d, 4H, d), 6.9 (d, 4H, e), 4.0 (t, 4H, f), 1.9 (m, 4H, g). 13C-NMR (500 MHz, DMSO-d6, ppm): C1-109.1, C2-156.8, C3-115.9, C4-131.1, C5-135.9, C6-159.9, C7-143.3, C8-122.6, C9-116.8, C10-156.3, C11-67.8, C12-28.7.
N), 834 (p-substituted aromatic). 1H-NMR (DMSO-d6, ppm): 6.9 (d, 4H, a), 7.6 (d, 4H, b), 8.6 (s, 2H, c), 7.1 (d, 4H, d), 6.9 (d, 4H, e), 4.0 (t, 4H, f), 1.9 (m, 4H, g). 13C-NMR (500 MHz, DMSO-d6, ppm): C1-109.1, C2-156.8, C3-115.9, C4-131.1, C5-135.9, C6-159.9, C7-143.3, C8-122.6, C9-116.8, C10-156.3, C11-67.8, C12-28.7.
2.5.2. 1,5-Bis[4(4-cyanatophenylazomethyl)phenoxy]pentane (CE2). Yield: 87%, m.p.: 99 °C. FT-IR (KBr, cm−1): 2226, 2240 (OCN), 2942 (OCH2), 1688 (HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 835 (p-substituted aromatic). 1H-NMR (DMSO-d6, ppm): 7.1 (d, 4H, a), 7.7 (d, 4H, b), 8.7 (s, 2H, c), 7.2 (d, 4H, d), 6.9 (d, 4H, e), 4.1 (t, 4H, f), 1.8 (m, 4H, g), 1.6 (m, 2H, h). 13C-NMR (500 MHz, DMSO-d6, ppm): C1-109.5, C2-155.8, C3-116.5, C4-131.1, C5-135.8, C6-161.1, C7-144.1, C8-121.9, C9-115.1, C10-157.1, C11-68.9, C12-29.6, C13-22.1.
N), 835 (p-substituted aromatic). 1H-NMR (DMSO-d6, ppm): 7.1 (d, 4H, a), 7.7 (d, 4H, b), 8.7 (s, 2H, c), 7.2 (d, 4H, d), 6.9 (d, 4H, e), 4.1 (t, 4H, f), 1.8 (m, 4H, g), 1.6 (m, 2H, h). 13C-NMR (500 MHz, DMSO-d6, ppm): C1-109.5, C2-155.8, C3-116.5, C4-131.1, C5-135.8, C6-161.1, C7-144.1, C8-121.9, C9-115.1, C10-157.1, C11-68.9, C12-29.6, C13-22.1.
2.5.3. 1,6-Bis[4(4-cyanatophenylazomethyl)phenoxy]hexane (CE3). Yield: 93%, m.p.: 123 °C. FT-IR (KBr, cm−1): 2219, 2242 (OCN), 2938 (OCH2), 1680 (HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 838 (p-substituted aromatic). 1H-NMR (DMSO-d6, ppm): 7.0 (d, 4H, a), 7.7 (d, 4H, b), 8.7 (s, 2H, c), 7.2 (d, 4H, d), 6.9 (d, 4H, e), 4.0 (t, 4H, f), 1.7 (m, 4H, g), 1.4 (m, 4H, h). 13C-NMR (500 MHz, DMSO-d6, ppm): C1-109.4, C2-156.3, C3-116.2, C4-131.9, C5-135.1, C6-160.1, C7-143.8, C8-121.8, C9-115.7, C10-157.9, C11-68.5, C12-29.6, C13-25.9.
N), 838 (p-substituted aromatic). 1H-NMR (DMSO-d6, ppm): 7.0 (d, 4H, a), 7.7 (d, 4H, b), 8.7 (s, 2H, c), 7.2 (d, 4H, d), 6.9 (d, 4H, e), 4.0 (t, 4H, f), 1.7 (m, 4H, g), 1.4 (m, 4H, h). 13C-NMR (500 MHz, DMSO-d6, ppm): C1-109.4, C2-156.3, C3-116.2, C4-131.9, C5-135.1, C6-160.1, C7-143.8, C8-121.8, C9-115.7, C10-157.9, C11-68.5, C12-29.6, C13-25.9.
2.6. Preparation of cyanate ester/epoxy blends
1 wt% of cyanate ester (0.200 g) was taken with 99 wt% of epoxy resin (19.80 g) (cyanate ester![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) epoxy = 1
epoxy = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99) and stirred at 80 °C until the mixture became homogeneous. 5.346 g (27 g of DDM taken for 100 g epoxy resin) of DDM (a hardener) was added to the homogeneous mixture of cyanate/epoxy resin and the mixture was immediately transferred to the mold and cured at 120 °C for 1 h, 170 °C for 1 h and post cured at 210 °C for 1 h. The same procedure was adopted for the preparation of cyanate/epoxy blends with 3% and 5% of cyanate ester.
99) and stirred at 80 °C until the mixture became homogeneous. 5.346 g (27 g of DDM taken for 100 g epoxy resin) of DDM (a hardener) was added to the homogeneous mixture of cyanate/epoxy resin and the mixture was immediately transferred to the mold and cured at 120 °C for 1 h, 170 °C for 1 h and post cured at 210 °C for 1 h. The same procedure was adopted for the preparation of cyanate/epoxy blends with 3% and 5% of cyanate ester.
3. Results and discussion
The synthetic procedure for the preparation of dicyanate esters is presented in Scheme 1. The bisphenol precursors were prepared by the condensation reaction between aromatic aldehydes and a diamine. The structure of the bisphenols was confirmed by FT-IR, 1H-NMR, and 13C-NMR spectroscopy. The dicyanate esters were prepared from their precursor diols and CNBr in the presence of triethylamine. Fig. 1 shows the FT-IR spectra of the dicyanate esters. The appearance of new bands at around 1680 cm−1 and the disappearance of amine bands (around 3300–3400 cm−1) confirmed the formation of the azomethine. The appearance of new bands at 2174–2240 cm−1 and disappearance of the band at 3300–3500 cm−1 due to hydroxyl group in the FT-IR spectrum confirms the conversion of hydroxyl group into a dicyanate ester. The formation of a C–O–C linkage is confirmed by the presence of the band at 1050 to 1070 cm−1.30 The signal at 3.7 ppm, due to the amino protons, disappears and a new signal at 8.7 ppm due to azomethine proton is formed indicating the conversion of the amino group to azomethine group. This is further supported by FT-IR spectrum. The strong absorption band due to the amino is missing and a new band is found at 1681 cm−1.
 |
| | Scheme 1 Synthesis of the dicyanate ester monomers. | |
 |
| | Fig. 1 FT-IR spectrum of the cyanate esters CE1, CE2 and CE3. | |
The conversion of the hydroxyl group to the cyanate ester is confirmed by the disappearance of the hydroxyl proton signal at 9.4 ppm and the appearance of the signal due to the cyanate ester carbon at 109.1 ppm13 from the NMR spectra (Fig. 2 and 3). The chemical shifts of all the aromatic protons appear in the range of 6.9 to 7.6 ppm. The chemical shift at 4.0 ppm may be attributed to the methylene proton which is attached to aromatic ring through an oxygen atom. The chemical shift at 1.9 ppm may be assigned to methylene protons which are present in the spacer group between the two aromatic rings. In the 13C-NMR spectrum, the carbon atoms in the azomethine linkage of the three cyanate esters show chemical shifts in the range of 115–116 ppm. Aromatic carbons appear in the range of 115.8–156.3 ppm. Hence, the proposed structure of the dicyanate ester is confirmed.
 |
| | Fig. 2 1H-NMR spectrum of the cyanate ester (CE1). | |
 |
| | Fig. 3 13C-NMR spectrum of cyanate ester (CE1). | |
3.1. DSC analysis
The cure behavior of the dicyanate esters was studied using differential scanning calorimetry. The DSC curves of the dicyanate esters CE1, CE2 and CE3 are shown in Fig. 4(a–c) and the data are given in Table 1. The melting points of the dicyanate esters were found to be 91, 102 and 123 °C respectively. The initial (Ti) and maximum curing temperatures (Tmax) were found to be in the range of 143 to 181 °C and 203 to 242 °C respectively. The cyanate ester CE3 (1,6-bis[4(4-cyanatophenylazomethyl)phenoxy]hexane) shows the peak maximum curing temperature. The end cure temperatures were found to be in the range of 246 to 285 °C. The dicyanate ester CE1 (1,4-bis[4(4-cyanatophenylazomethyl)phenoxy]butane) showed the highest end cure temperature of 285 °C. The curing temperature of BADCY is found to be at 281 °C2 and all of the three synthesised cyanate esters were cured at a lower temperature when compared with BADCY. The cyanate ester (CE1) with shortest alkyl chain between the aromatic groups was found to cure at the lowest temperature. The glass transition temperatures of the neat cured dicyanate esters decreased with increasing alkyl chain length between the two aromatic groups. No liquid crystallinity was observed for any of the dicyanate esters.
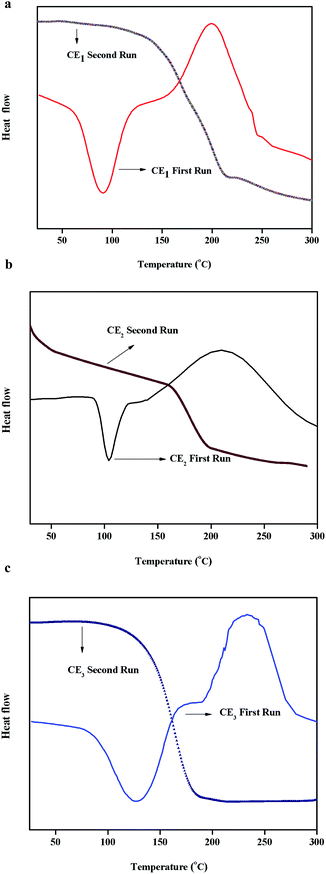 |
| | Fig. 4 (a) DSC thermogram data of the cyanate ester CE1, (b) DSC thermogram data of the cyanate ester CE2 and (c) DSC thermogram data of the cyanate ester CE3. | |
Table 1 DSC data of the cyanate esters
| Sample code |
Melting point (°C) |
Onset of cure (°C) |
Maximum cure (°C) |
End of cure (°C) |
| CE1 |
91 |
148 |
203 |
285 |
| CE2 |
102 |
143 |
216 |
246 |
| CE3 |
123 |
181 |
242 |
269 |
3.2. Thermogravimetric analysis
Thermogravimetric analysis (TGA) is the preferred technique for the evaluation of the thermal stability of the polymeric materials. The thermal stability of the cured cyanate esters was evaluated with its 5% weight loss temperature (T5%), 10% weight loss temperatures (T10%) and the char yield at 800 °C. The TGA curves of the various cured cyanate esters are given in Fig. 5 and the data are given in Table 2. T5% and T10% of all three neat cured cyanate esters were found to be in the range of 358 to 465 °C and 508 to 525 °C respectively. The cured cyanate ester (CE2) with an alkyl chain length of five showed the least thermal stability (T5% – 358 °C) and lost 40% weight between 520 to 593 °C. The char yield was found to be in the range of 37.06 to 57.26%. From the char yield and LOI values, the cyanate esters (CE1 and CE3) with an even number alkyl chain length between the two aromatic groups were more stable than the dicyanate ester (CE2) with an odd number spacer length between the two aromatic groups. The char yield of BADCY is found to be 42.5 at 800 °C,2 but CE1 and CE3 show higher char yields of 57.26 and 48.08 compared to the commercially available dicyanate ester (BADCY). The cured cyanate ester with the shortest alkyl chain length between the two aromatic groups show 5% weight loss at 465 °C. This is greater than that of other two cured cyanate esters. The 5% weight CE/epoxy blends show 10% (T10%) weight loss temperatures in the range of 392–410 °C and char yields in the range of 24.11 to 31.26%. Few reports by other authors that the thermal stability and char yield of cyanate ester/epoxy blends increase with increasing concentration of the cyanate ester in the epoxy blends, which is similar to our case.13
 |
| | Fig. 5 TGA thermogram data of the cyanate esters. | |
Table 2 TGA data of the polycyanuratesa
| Sample code |
T5 (°C) |
T10 (°C) |
Char yield% |
LOI |
| T5 = 5% weight loss temperature, T10 = 10% weight loss temperature, LOI = limiting oxygen index. |
| CE1 |
465 |
508 |
57.26 |
40.40 |
| CE2 |
358 |
520 |
37.06 |
32.32 |
| CE3 |
399 |
525 |
48.08 |
36.73 |
| Neat epoxy |
363 |
369 |
18.0 |
24.7 |
| 5% CE1 + 95% epoxy |
393 |
410 |
31.26 |
30.00 |
| 5% CE2 + 95% epoxy |
378 |
392 |
24.11 |
27.14 |
| 5% CE3 + 95% epoxy |
381 |
395 |
27.80 |
28.62 |
3.3. SEM analysis
The cross-section (fracture surface) of the CE/epoxy blends was studied by scanning electron microscopy. The fractured surface of the neat CE and CE/epoxy blends was sputtered with gold to deionize the electrons on the surface. Fig. 6(a–c) display the SEM images of the cured neat cyanate ester CE1, 1% cyanate ester + 99% epoxy and 5% cyanate ester + 95% epoxy respectively. The SEM images of the fractured surface of CE2 and CE3 are given in Fig. 6(d and e) respectively. CE1 shows a curved hollow structure, CE2 and CE3 show a layered structure (on both lower and higher magnification). SEM images Fig. 6(b and c) illustrate the uniform distribution of the cyanate ester in the epoxy blends without any voids. The DMA results show that the toughening of epoxy was affected by adding CE. The neat CE2 has a similar morphology to the polycyanurate of bisphenol A dicyanate ester.31
 |
| | Fig. 6 SEM images of (a) neat cyanate ester CE1, (b) 1% cyanate ester CE1 + 99% epoxy system, (c) 5% cyanate ester CE1 + 95% epoxy system, (d) neat cyanate ester CE2 and (e) neat cyanate ester CE3. | |
3.4. Dynamic mechanical analysis (DMA)
The dynamic mechanical properties (stress relaxation behavior) were measured for the CE/epoxy blend systems in order to find out the effect of increasing the cyanate ester content. The storage modulus and tan delta of the blend with 5% cyanate ester (CE1 CE2 and CE3) and 95% epoxy are given in Fig. 7 and 8 respectively. The data obtained from the DMA scans are given in Table 3. The storage modulus of the CE/epoxy blends increases with the increase in the cyanate ester content (from 1 to 5%) except the 1% CE1/epoxy blend. The increase in the storage modulus may be due to the increased cross-linking density of the blends as a result of increase in the CE content. The glass transition temperature of the pure epoxy system was found to be 161 °C.13 In the case of the 1% CE1 epoxy blend, the glass transition temperature decreased to 158 °C from 161 °C for the neat cured epoxy. However, on further addition of 5% CE1 to the epoxy resin, the glass transition temperature increased to 180 °C from 161 °C. The glass transition temperature of the blends slightly increased with increase in the cyanate ester content. This observation proves the fact that the co-reaction of cyanate ester with epoxy resin is almost complete resulting in a homogeneous state14 and it is the resultant oxazolidinone network25 which enhances the thermal and mechanical properties of the CE/epoxy blends. However, some authors reported that the addition of POSS-CY to the epoxy resin leads to a decrease in the glass transition temperature from 161 to 155 °C due to the formation of free volume in cyanate/epoxy blend system.13
 |
| | Fig. 7 DMA showing storage modulus (E′) of the CE/epoxy blends: (a) 5% of CE1 + 95% epoxy, (b) 5% of CE2 + 95% epoxy, (c) 5% of CE3 + 95% epoxy. | |
 |
| | Fig. 8 DMA showing loss modulus of the CE/epoxy blends: (a) 5% of CE1 + 95% epoxy, (b) 5% of CE2 + 95% epoxy, (c) 5% of CE3 + 95% epoxy. | |
Table 3 Values of the crosslink density of the CE + epoxy blends (from the DMA values)
| Sample code |
CE% |
E′ (GPa) |
Temp. K (273 + 50) |
Crosslink density νe × 105 (mol m−3) |
| CE1 + epoxy |
5 |
2.52 |
323 |
3.13 |
| CE2 + epoxy |
5 |
3.48 |
323 |
4.32 |
| CE3 + epoxy |
5 |
2.09 |
323 |
2.59 |
3.5. Crosslink density
The crosslink density, or the concentration of network chains (νe), is the number of moles of network chains per unit volume of the cured polymers. The crosslink density of highly crosslinked thermosets can be determined by modulus measurements in the rubbery plateau by using the equation of state for rubber elasticity:32
where E′ = tensile storage modulus in the rubbery plateau, T = temperature in K corresponding to the storage modulus value and R = gas constant.
Table 3 shows the crosslink density for each of the CE/epoxy blends. On addition of cyanate ester to the epoxy resin, the crosslink density increased. The CE2 epoxy blends showed the highest crosslink density. The storage modulus of CE2 blends (3.48 GPa), with an odd number alkyl chain length between aromatic groups, is higher than the CE/epoxy blends which have an even number alkyl chain length between the two aromatic groups, CE1 (2.52 GPa) and CE3 (2.09 GPa).
3.6. Flame retardancy
The resistance towards fire of the CEs and the CE/epoxy blends is expressed in terms of the limiting oxygen index (LOI). LOI may be defined as the minimum fraction of oxygen in a mixture of O2 and N2 during a fire. The LOI value should be higher than that of a threshold value (a value of 26) for the polymer to behave as a self-extinguishing material or a self-extinguisher. The LOI value is calculated using Krevelen's equation:33
where σ is the percentage of char yield. The LOI of the neat CE and CE/epoxy blends were found to be in the range of 32.32 to 40.40 and 27.14 to 30.00 respectively. The cured cyanate ester with the least alkyl space length (CE1) showed the highest LOI value and the cyanate ester (CE2) with an alkyl chain of five carbons showed the lowest LOI value. The LOI values of all the cured dicyanate esters indicate that they are very good fire retardant materials. From the LOI values, it was found that the cured cyanate ester CE1/epoxy blends showed the best flame retardance.
4. Conclusions
Dicyanate esters with azomethine linkages were successfully synthesised. The structure of the monomers and precursors were characterized by FT-IR, 1H-NMR and 13C-NMR spectroscopy, which clearly confirmed the molecular structure. The morphology of the cured cyanate ester and CE/epoxy blends revealed the uniform distribution of cyanate ester in the epoxy resin. The cured cyanate ester and BADCY showed the similar morphologies. The DMA studies revealed that the glass transition temperature of the epoxy was increased from 161 to 180 °C and the storage modulus of the epoxy resin increased with increases in cyanate ester content. The crosslink density of the cyanate epoxy blends were calculated from their storage modulus value, which increased (from 3.98 to 4.32 mol m−3 for CE2) with increases in the weight ratio of CE in the blends. TGA results showed that the char yield values of neat cyanate esters are in the range of 32.32 to 40%. The char yield increased from 18% to 30.0% with increases of CE content in the epoxy blend. This increase in char yield further suggests that the CE/epoxy blends can be used in high flame retardancy applications. The microwave curing and the effect of odd and even alkyl chain length between the two aromatic groups will be studied in future, as CE1 and CE3, which have an even number alkyl chain length between the two aromatic rings, showed better char yield and LOI values compared to CE2 which has an odd number alkyl chain length between the two aromatic rings.
Acknowledgements
This work was supported by the University Grants Commission (UGC), India and the Natural Sciences and Engineering Research Council (NSERC) of Canada.
References
- C. Dreyer, D. Söthje and M. Bauer, Adv. Chem. Eng. Sci., 2014, 4, 167–183 CrossRef CAS.
- S. Dai, D. Zhuo, A. Gu, G. Liang and L. Yuan, Polym. Eng. Sci., 2011, 51, 2236–2244 CAS.
- Y. Shen, A. Gu, G. Liang and L. Yuan, Composites, Part A, 2010, 41, 1668–1676 CrossRef PubMed.
- X.-Y. Zhao and M.-Z. Wang, Plasma Chem. Plasma Process., 2012, 33, 237–247 CrossRef.
- X. Zhao, M. Wang, Z. Sun, C. Niu, J.-J. Xiao and E.-J. Tang, Plasma Processes Polym., 2012, 9, 468–472 CrossRef CAS.
- A. J. Guenthner, G. R. Yandek, M. E. Wright, B. J. Petteys, R. Quintana, D. Connor, R. D. Gilardi and D. Marchant, Macromolecules, 2006, 39, 6046–6053 CrossRef CAS.
- S. He, G. Liang, J. Wang and H. Yan, Polym. Bull., 2008, 62, 237–246 CrossRef PubMed.
- B. Wang, Y. Jiao, A. Gu, G. Liang and L. Yuan, Compos. Sci. Technol., 2014, 91, 8–15 CrossRef CAS PubMed.
- X. Sheng, R. Hanus, A. Bauer and M. R. Kessler, J. Appl. Polym. Sci., 2013, 130, 463–469 CrossRef CAS.
- J. Li, Z. Wu, C. Huang, H. Liu, R. Huang and L. Li, Compos. Sci. Technol., 2014, 90, 166–173 CrossRef CAS PubMed.
- Y. Huang, X. Ma, X. Wang, X. He and L. Liu, Mater. Chem. Phys., 2010, 121, 241–248 CrossRef CAS PubMed.
- Y. Tada, N. Moriya, T. Inoue, A. Suzuki and S. Koyama, Phosphorus, Sulfur Silicon Relat. Elem., 2012, 187, 1555–1567 CrossRef CAS.
- S. Rakesh, C. P. S. Dharan, M. Selladurai, V. Sudha, P. R. Sundararajan and M. Sarojadevi, High Perform. Polym., 2012, 25, 87–96 CrossRef PubMed.
- Z. Zhang, G. Liang and X. Wang, Polym. Int., 2014, 63, 552–559 CrossRef CAS.
- Y. Tang, J. Gu, Y. Yu and J. Kong, Polym. Compos., 2014 DOI:10.1002/pc.23111.
- A. Idesaki, H. Uechi, Y. Hakura and H. Kishi, Radiat. Phys. Chem., 2014, 98, 1–6 CrossRef CAS PubMed.
- P. Brahmbhatt, J. Unnikrishnan, J. D. Sudha and S. Pradhan, J. Appl. Polym. Sci., 2012, 125, 1068–1076 CrossRef CAS.
- R. Biju, C. Gouri and C. P. Reghunadhan Nair, Eur. Polym. J., 2012, 48, 499–511 CrossRef CAS PubMed.
- C. H. Lin, Polymer, 2004, 45, 7911–7926 CrossRef CAS PubMed.
- G. Liang, P. Ren, Z. Zhang and T. Lu, J. Appl. Polym. Sci., 2006, 101, 1744–1750 CrossRef CAS.
- H.-J. Hwang, C.-H. Li and C.-S. Wang, Polymer, 2006, 47, 1291–1299 CrossRef CAS PubMed.
- I. Hamerton, B. J. Howlin, S. L. Jewell and P. Patel, React. Funct. Polym., 2012, 72, 279–286 CrossRef CAS PubMed.
- N. J. Suman, J. Reinf. Plast. Compos., 2005, 24, 1105–1114 CrossRef CAS PubMed.
- A. Bauer, M. Thunga, K. Obusek, M. Akinc and M. R. Kessler, Polymer, 2013, 54, 3994–4002 CrossRef CAS PubMed.
- X. Li, Y. Xia, W. Xu, Q. Ran and Y. Gu, Polym. Chem., 2012, 3, 1629 RSC.
- K. S. Santhosh Kumar, C. P. Reghunadhan Nair and K. N. Ninan, Eur. Polym. J., 2009, 45, 494–502 CrossRef PubMed.
- B. Francis, V. L. Rao, G. Vanden Poel, F. Posada, G. Groeninckx, R. Ramaswamy and S. Thomas, Polymer, 2006, 47, 5411–5419 CrossRef CAS PubMed.
- G. Anuradha and M. Sarojadevi, Polym. Compos., 2009, 30, 782–790 CrossRef.
- M. Ariraman, R. Sasi and M. Alagar, J. Appl. Polym. Sci., 2014, 41097, 1–10 Search PubMed.
- P. A. Henderson, R. T. Inkster, M. Seddon and C. T. Imrie, J. Mater. Chem., 2001, 2722–2731 RSC.
- L. Yuan, A. Gu and G. Liang, Polym. Compos., 2008, 31, 136–144 Search PubMed.
- L. W. Hill, Prog. Org. Coat., 1997, 31, 235–243 CrossRef CAS.
- D. W. Van Krevelen, Polymer, 1975, 16, 615–620 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 824 (p-substituted aromatic).
O), 824 (p-substituted aromatic).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 834 (p-substituted aromatic). 1H-NMR (DMSO-d6, ppm): 9.4 (s, 2H, a), 6.9 (d, 4H, b), 7.7 (d, 4H, c), 8.7 (s, 2H, d), 7.1 (d, 4H, e), 6.9 (d, 4H, f), 4.1 (t, 4H, g), 1.9 (m, 4H, h). 13C-NMR (500 MHz, DMSO-d6, ppm): C1-161.1, C2-116.8, C3-130.9, C4-130.1, C5-159.3, C6-145.9, C7-122.3, C8-116.8, C9-155.8, C10-66.3, C11-27.8.
N), 834 (p-substituted aromatic). 1H-NMR (DMSO-d6, ppm): 9.4 (s, 2H, a), 6.9 (d, 4H, b), 7.7 (d, 4H, c), 8.7 (s, 2H, d), 7.1 (d, 4H, e), 6.9 (d, 4H, f), 4.1 (t, 4H, g), 1.9 (m, 4H, h). 13C-NMR (500 MHz, DMSO-d6, ppm): C1-161.1, C2-116.8, C3-130.9, C4-130.1, C5-159.3, C6-145.9, C7-122.3, C8-116.8, C9-155.8, C10-66.3, C11-27.8.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 836 (p-substituted aromatic). 1H-NMR (DMSO-d6, ppm): 9.2 (s, 2H, a), 6.8 (d, 4H, b), 7.8 (d, 4H, c), 8.6 (s, 2H, d), 7.2 (d, 4H, e), 7.0 (d, 4H, f), 4.0 (m, 4H, g), 1.8 (m, 4H, h), 1.8 (m, 2H, i). 13C-NMR (500 MHz, DMSO-d6, ppm): C1-160.5, C2-115.8, C3-131.2, C4-129.1, C5-159.9, C6-145.5, C7-122.5, C8-115.7, C9-158.5, C10-67.3, C11-29.8, C12-22.8.
N), 836 (p-substituted aromatic). 1H-NMR (DMSO-d6, ppm): 9.2 (s, 2H, a), 6.8 (d, 4H, b), 7.8 (d, 4H, c), 8.6 (s, 2H, d), 7.2 (d, 4H, e), 7.0 (d, 4H, f), 4.0 (m, 4H, g), 1.8 (m, 4H, h), 1.8 (m, 2H, i). 13C-NMR (500 MHz, DMSO-d6, ppm): C1-160.5, C2-115.8, C3-131.2, C4-129.1, C5-159.9, C6-145.5, C7-122.5, C8-115.7, C9-158.5, C10-67.3, C11-29.8, C12-22.8.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) 839 (p-substituted aromatic). 1H-NMR (DMSO-d6, ppm): 9.2 (s, 2H, a), 6.9 (d, 4H, b), 7.9 (d, 4H, c), 8.6 (s, 2H, d), 7.2 (d, 4H, e), 6.9 (d, 4H, f), 4.1 (t, 4H, g), 1.7 (m, 4H, h), 1.5 (m, 2H, i). 13C-NMR (500 MHz, DMSO-d6, ppm): C1-160.8, C2-116.1, C3-131.1, C4-128.9, C5-160.1, C6-144.8, C7-121.5, C8-115.9, C9-157.8, C10-68.9, C11-29.6, C12-25.8.
N) 839 (p-substituted aromatic). 1H-NMR (DMSO-d6, ppm): 9.2 (s, 2H, a), 6.9 (d, 4H, b), 7.9 (d, 4H, c), 8.6 (s, 2H, d), 7.2 (d, 4H, e), 6.9 (d, 4H, f), 4.1 (t, 4H, g), 1.7 (m, 4H, h), 1.5 (m, 2H, i). 13C-NMR (500 MHz, DMSO-d6, ppm): C1-160.8, C2-116.1, C3-131.1, C4-128.9, C5-160.1, C6-144.8, C7-121.5, C8-115.9, C9-157.8, C10-68.9, C11-29.6, C12-25.8.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 834 (p-substituted aromatic). 1H-NMR (DMSO-d6, ppm): 6.9 (d, 4H, a), 7.6 (d, 4H, b), 8.6 (s, 2H, c), 7.1 (d, 4H, d), 6.9 (d, 4H, e), 4.0 (t, 4H, f), 1.9 (m, 4H, g). 13C-NMR (500 MHz, DMSO-d6, ppm): C1-109.1, C2-156.8, C3-115.9, C4-131.1, C5-135.9, C6-159.9, C7-143.3, C8-122.6, C9-116.8, C10-156.3, C11-67.8, C12-28.7.
N), 834 (p-substituted aromatic). 1H-NMR (DMSO-d6, ppm): 6.9 (d, 4H, a), 7.6 (d, 4H, b), 8.6 (s, 2H, c), 7.1 (d, 4H, d), 6.9 (d, 4H, e), 4.0 (t, 4H, f), 1.9 (m, 4H, g). 13C-NMR (500 MHz, DMSO-d6, ppm): C1-109.1, C2-156.8, C3-115.9, C4-131.1, C5-135.9, C6-159.9, C7-143.3, C8-122.6, C9-116.8, C10-156.3, C11-67.8, C12-28.7.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 835 (p-substituted aromatic). 1H-NMR (DMSO-d6, ppm): 7.1 (d, 4H, a), 7.7 (d, 4H, b), 8.7 (s, 2H, c), 7.2 (d, 4H, d), 6.9 (d, 4H, e), 4.1 (t, 4H, f), 1.8 (m, 4H, g), 1.6 (m, 2H, h). 13C-NMR (500 MHz, DMSO-d6, ppm): C1-109.5, C2-155.8, C3-116.5, C4-131.1, C5-135.8, C6-161.1, C7-144.1, C8-121.9, C9-115.1, C10-157.1, C11-68.9, C12-29.6, C13-22.1.
N), 835 (p-substituted aromatic). 1H-NMR (DMSO-d6, ppm): 7.1 (d, 4H, a), 7.7 (d, 4H, b), 8.7 (s, 2H, c), 7.2 (d, 4H, d), 6.9 (d, 4H, e), 4.1 (t, 4H, f), 1.8 (m, 4H, g), 1.6 (m, 2H, h). 13C-NMR (500 MHz, DMSO-d6, ppm): C1-109.5, C2-155.8, C3-116.5, C4-131.1, C5-135.8, C6-161.1, C7-144.1, C8-121.9, C9-115.1, C10-157.1, C11-68.9, C12-29.6, C13-22.1.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 838 (p-substituted aromatic). 1H-NMR (DMSO-d6, ppm): 7.0 (d, 4H, a), 7.7 (d, 4H, b), 8.7 (s, 2H, c), 7.2 (d, 4H, d), 6.9 (d, 4H, e), 4.0 (t, 4H, f), 1.7 (m, 4H, g), 1.4 (m, 4H, h). 13C-NMR (500 MHz, DMSO-d6, ppm): C1-109.4, C2-156.3, C3-116.2, C4-131.9, C5-135.1, C6-160.1, C7-143.8, C8-121.8, C9-115.7, C10-157.9, C11-68.5, C12-29.6, C13-25.9.
N), 838 (p-substituted aromatic). 1H-NMR (DMSO-d6, ppm): 7.0 (d, 4H, a), 7.7 (d, 4H, b), 8.7 (s, 2H, c), 7.2 (d, 4H, d), 6.9 (d, 4H, e), 4.0 (t, 4H, f), 1.7 (m, 4H, g), 1.4 (m, 4H, h). 13C-NMR (500 MHz, DMSO-d6, ppm): C1-109.4, C2-156.3, C3-116.2, C4-131.9, C5-135.1, C6-160.1, C7-143.8, C8-121.8, C9-115.7, C10-157.9, C11-68.5, C12-29.6, C13-25.9.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) epoxy = 1
epoxy = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99) and stirred at 80 °C until the mixture became homogeneous. 5.346 g (27 g of DDM taken for 100 g epoxy resin) of DDM (a hardener) was added to the homogeneous mixture of cyanate/epoxy resin and the mixture was immediately transferred to the mold and cured at 120 °C for 1 h, 170 °C for 1 h and post cured at 210 °C for 1 h. The same procedure was adopted for the preparation of cyanate/epoxy blends with 3% and 5% of cyanate ester.
99) and stirred at 80 °C until the mixture became homogeneous. 5.346 g (27 g of DDM taken for 100 g epoxy resin) of DDM (a hardener) was added to the homogeneous mixture of cyanate/epoxy resin and the mixture was immediately transferred to the mold and cured at 120 °C for 1 h, 170 °C for 1 h and post cured at 210 °C for 1 h. The same procedure was adopted for the preparation of cyanate/epoxy blends with 3% and 5% of cyanate ester.









