A novel solid acid catalyst synthesized from ZnAl2O4 spinel and its application in the esterification of acetic acid and n-butyl alcohol
An-Qi Wang,
Xiu-Ling Wu,
Jun-Xia Wang*,
Hui Pan,
Xiao-Yun Tian and
Yu-Lin Xing
Faculty of Materials Science and Chemistry, Engineering Research Center of Nano-Geomaterials of Ministry of Education, China University of Geosciences, Wuhan 430074, China. E-mail: jxwyqh@sina.com; Fax: +86-27-87407079; Tel: +86-27-87407079
First published on 12th February 2015
Abstract
A new spinel-style S2O82−/ZnAl2O4 solid acid catalyst was prepared by sol–gel method. The catalytic performance was evaluated by the esterification of n-butyl acetate. The structure, morphology and acidity of the samples were characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), Field emission scanning electron microscopy (FE-SEM), N2 physisorption, X-ray photoelectron spectroscopy (XPS) and NH3 temperature programmed desorption (NH3-TPD). The results showed that the catalytic performances were significantly affected by the calcination temperature. S2O82−/ZnAl2O4-600 exhibited the highest catalytic activity of 91.7% under the optimum reaction conditions. The characterization showed that the mesoporous structure S2O82−/ZnAl2O4-600 maintained the well crystallinity of spinel as well as the spinel-style carrier in the sulfidation and recycling.
1. Introduction
The esterification of carboxylic acids with alcohols is an essential reaction in organic synthesis, pharmaceuticals, food preservatives, biofuels, etc. Two representative catalysts, SO42−/ZrO2 and SO42−/TiO2, are widely used in these esterifications, due to their strong acidity, non-toxicity and high catalytic activity.1,2 However, they have a similar problem in the crystal structure transformation in the process of synthesis and modification.3,4 It is well known that the crystal structure of metal oxide has a great influence on the catalytic performances.5 Therefore, it is desirable for researchers to look for a stable carrier to synthesize solid acid.Spinel-type oxide has drawn great attention for catalytic reactions because of its desirable properties, such as single crystal shape, high thermal stability and well diffusion, etc.6–9 A nanosized sulfated ZnFe2O4 catalyst with highly ordered mesoporous was found to be highly active towards commercially important fine chemicals.10 The high specific surface area SO42−/CoFe2O4 solid acid with the spinel as carrier displayed the excellent activity in the synthesis of ethyl acetate.11 Nevertheless, the literature on the catalytic applications of S2O82−/ZnAl2O4 catalyst is scanty. In our previous studies, the composite catalysts of Al-based catalysts have been developed and studied in terms of the catalytic activity and reusability, which performed the high catalytic activity and excellent stability.12–14 Therefore, the ZnAl2O4 spinel may be a promising carrier for solid acid.
In this work, a series of S2O82−/ZnAl2O4-T were prepared and evaluated by the esterification of n-butyl acetate. The influences of synthesis conditions on the catalytic activities were studied. In addition, the reaction conditions and catalyst recyclability were also performed in detail. Furthermore, the structure, morphology and acidity of the catalyst were characterized by XRD, FE-SEM, N2 physisorption, FT-IR, NH3-TPD and XPS techniques. Some valuable evaluations for the S2O82−/ZnAl2O4 in this paper have some referential value in the design of a novel ZnAl2O4 carrier for SO42−/MxOy solid acid synthesis.
2. Experimental
2.1 Catalysts preparation
S2O82−/ZnAl2O4 solid acid catalysts were prepared by the following steps: 0.04 mol Al(NO3)3·9H2O and 0.02 mol Zn(NO3)2·6H2O were dissolved in 20 mL ethyl alcohol, followed by adding 5 wt% (the total weight of nitrates) polyethylene glycol (PEG-2000) into the ethyl alcohol solution with magnetic stirring for 4 h at room temperature. Afterward, the obtained mixture was evaporated for 1 h at 60 °C to obtain the sol. The sol was dried and then grounded into a fine powder. The fine powder was calcined at 400 °C, 500 °C, 550 °C, 600 °C and 650 °C for 5 h in air to obtain the precursors. The obtained precursors with different calcination temperature were designated as ZnAl2O4-T. The ZnAl2O4-T were then sulfated for 12 h by impregnating with 0.75 mol L−1, 1.00 mol L−1, 1.25 mol L−1, 1.50 mol L−1, 1.75 mol L−1 and 2.00 mol L−1 (NH4)2S2O8 solution. The sulfidation was done by impregnating 1 g of ZnAl2O4 in 10 mL of (NH4)2S2O8 solution for 12 hours. After having been filtered and dried, the solids were calcined at 550 °C for 5 h in air to obtain the S2O82−/ZnAl2O4-T catalysts. The S2O82−/ZnO-600 and S2O82−/Al2O3-600 catalysts were synthesized by the same steps.2.2 Catalysts characterization
The X-ray diffraction (XRD) was conducted on a Bruker AXS D8-Focus X diffractometer, using CuKα radiation at 40 kV and 40 mA; The Fourier transform infrared spectroscopy (FT-IR) spectra of the catalysts were recorded in the range of 400–4000 cm−1 by a Nicolet 6700 IR spectrometer using the KBr pellet technique; the morphology was examined by using Field emission scanning electron microscopy (FE-SEM) on a SU 8010 electron microscope; the X-ray photoelectron spectroscopy (XPS) was performed on a VG Multilab 2000; the NH3 temperature programmed desorption (NH3-TPD) experiment was carried out using a Micromeritics AutoChem II 2920 quipped with a TCD detector; N2 adsorption analysis was carried out using micromeritics (ASAP-2020) at liquid nitrogen temperature (77 K). The surface area was calculated by the BET method and the pore size distribution was obtained from the adsorption isotherm by the BJH method.2.3 Catalytic activity test
The esterification efficiency of n-butyl acetate was evaluated in a 250 mL three-necked flask equipped with a magnetic stirrer, a thermometer and a refluxing condenser. The mixture was stirred at about 118 °C under atmospheric pressure. Various parameters of the reaction conditions, including the molar ratio of n-butanol to acetic acid, catalyst amount (percentage content of the reaction mixture) and reaction time, were varied to the optimum. The initial acid and residual acid were detected by means of titration with 0.10 mol L−1 NaOH solution. The esterification efficiency of acetic acid can be calculated using the following equation (by the method of GB1668-81):where M0 is the acid value before reaction and M1 is the acid value after reaction.
3. Results and discussion
3.1 Characterization of catalysts
Fig. 1(a) shows the XRD patterns of ZnAl2O4-T and S2O82−/ZnAl2O4-T. It reveals that the calcination temperatures cause apparently changes in the crystalline structure of the carriers. The ZnAl2O4-400, ZnAl2O4-500 and ZnAl2O4-550 are mainly consisted of amorphous phase. When the calcinations temperature further increases, the amorphous gradually transforms into spinel. The characteristic peaks at 2θ = 18.9°, 31.4°, 36.90°, 44.2°, 49.2°, 55.8°, 59.5° and 65.4° are observed in ZnAl2O4-600 and ZnAl2O4-650, which are corresponding to the crystal planes of the spinel structure (JCPDS File no. 05-0669). Similar peaks are observed in S2O82−/ZnAl2O4-600 and S2O82−/ZnAl2O4-650, indicating that the spinel carriers have the excellent advantage of structure stability in sulfidation. Compared with the carriers, the crystallite size of S2O82−/ZnAl2O4-T is larger than ZnAl2O4-T as shown in Table 1. This result may be that the high calcination temperature in the process of sulfidation causes crystalline grain further to grow. Meanwhile, it can be found that the crystallite size gradually increases with increasing temperature. Besides, ZnSO4·H2O and Al2(SO4)3 are observed in sulfated catalyst, which may be due to the long-time impregnation and the interaction between excess S2O82− and the metal ions.15,16 Correlating the crystallization with the following catalytic activities, the result shows that the spinel is an available carrier for solid acid.| Crystallite sizea (nm) | |||||
|---|---|---|---|---|---|
| 400 | 500 | 550 | 600 | 650 | |
| a From Debye–Scherrer equation using the width (at half maximum) of [3 1 1] line.b The synthesis condition: calcination temperature was 600 °C; the concentration of (NH4)2S2O8 solution was 1.50 mol L−1. | |||||
| ZnAl2O4-T | — | — | 7.23 | 17.99 | 25.82 |
| S2O82−/ZnAl2O4-Tb | 5.83 | 8.69 | 19.28 | 20.72 | 27.62 |
Fig. 1(b) clearly shows the XRD patterns of S2O82−/ZnAl2O4-600 with different impregnation concentration. It is noteworthy that all the catalysts appear the strong diffraction peaks of spinel (JCPDS File no. 05-0669). The well crystallization indicates that the spinel carrier perform the advantages of single crystal shape and stable structure with different impregnation concentration. Besides, the XRD patterns of used S2O82−/ZnAl2O4-600 in Fig. 1(b) show no change in the crystallinity by comparing to the fresh catalyst, indicating the stable structure in recycling test.
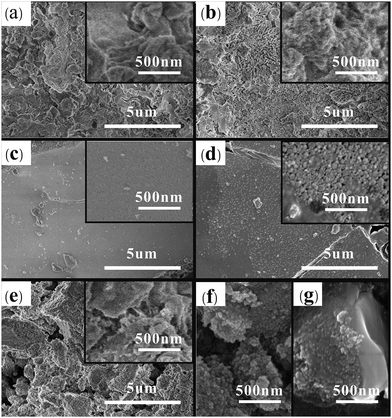
FE-SEM images in Fig. 2 display the S2O82−/ZnAl2O4-T with different calcination temperatures. The irregular agglomerations are apparently appeared on the surfaces of S2O82−/ZnAl2O4-400, S2O82−/ZnAl2O4-500 and S2O82−/ZnAl2O4-650, which may be one reason of their low catalytic activities. The S2O82−/ZnAl2O4-550 and S2O82−/ZnAl2O4-600 show the morphology with flat blocks. By comparison, S2O82−/ZnAl2O4-600 is composed of porous structures, which is assembled by numerous nanoparticles. Similarly, the fresh S2O82−/ZnAl2O4-600 (Fig. 2(f)), impregnated with 1.50 mol L−1 (NH4)2S2O8 solution, performs the porous structures. Correspondingly, the specific surface area, mean pore diameter and pore volumes are investigated by N2 adsorption analysis in Fig. 3. The N2 adsorption/desorption isotherms correspond to the IV isotherm with a hysteresis loop in the low relative (P/P0) range of 0.4–1, which are typical for mesoporous materials. The surface area and average pore diameter are 18.776 m2 g−1 and 8.864 nm, respectively. Similar porous structures can be observed in high active SO42−/TiO2 catalysts.5 These porous structures of S2O82−/ZnAl2O4-600 may be beneficial to its high catalytic activity. Besides, the used S2O82−/ZnAl2O4-600 catalyst in Fig. 2(g) maintains the agglomerate nanoparticles as the fresh catalyst.
 | ||
| Fig. 3 N2 adsorption–desorption isotherms and pore-size distributions of S2O82−/ZnAl2O4-600 (impregnated with 1.50 mol L−1 (NH4)2S2O8 solution). | ||
Fig. 4 records the FT-IR spectra of the ZnAl2O4-600 carrier, S2O82−/ZnAl2O4-600 solid acid catalyst and recycling catalyst. It can discern that three bands (around 672, 556 and 493 cm−1) appeared in all the samples are consistent with characteristics of Al–O stretching vibrations, Zn–O stretching vibrations and Al–O bending vibrations in the crystal structure of ZnAl2O4 spinel, respectively.17 The result confirms that the spinel structure is formed in all the prepared samples, which is in agreement with the XRD analysis. Compared with ZnAl2O4-600 carrier, there are distinguishing absorption peaks between 900 and 1400 cm−1 in sulfated catalyst and recycling catalyst. The additional absorption peak characterizes the sulfate groups on the surface which are related to catalytic activity. The three bands at 982, 1103 and 1145 cm−1 are assigned to the stretching vibration of S–O. The bands at 1220 and 1398 cm−1 are assigned to the stretching vibration of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.18 The existences of S
O.18 The existences of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and S–O bonds contribute to the coordination of the inorganic chelating bidentate sulfate ion with surface metal cation.19 The recycling catalyst also displays the similar peaks belonged to spinel structure and sulfate groups, indicating that the S2O82−/ZnAl2O4-600 catalyst has the advantage of structure stability in recycling test. This result is in agreement with the XRD analysis. In addition, the band around 1627 cm−1 is assigned to the bending modes of the –OH group.
O and S–O bonds contribute to the coordination of the inorganic chelating bidentate sulfate ion with surface metal cation.19 The recycling catalyst also displays the similar peaks belonged to spinel structure and sulfate groups, indicating that the S2O82−/ZnAl2O4-600 catalyst has the advantage of structure stability in recycling test. This result is in agreement with the XRD analysis. In addition, the band around 1627 cm−1 is assigned to the bending modes of the –OH group.
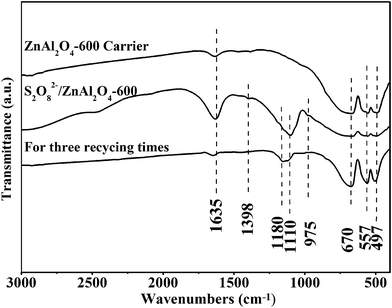 | ||
| Fig. 4 FT-IR spectra of ZnAl2O4-600 carrier, fresh and used S2O82−/ZnAl2O4-600 catalysts (impregnated with 1.50 mol L−1 (NH4)2S2O8 solution). | ||
As shown in Fig. 5(a), the acid strength distributions of S2O82−/ZnAl2O4-600 and reused catalysts are characterized by the NH3-TPD. The higher of desorption peak temperature represents the stronger of the acid strength. The fresh S2O82−/ZnAl2O4-600 catalyst shows prominent broad peaks in the range of 100 °C to 700 °C, indicating a wide distribution of acidic sites. The two peaks around 190 °C and 350 °C correspond to the acidic site of weak and medium strength, respectively.20 The peak above 500 °C assigns to strong acidic site.21 The area of the NH3 desorption curve shown in Fig. 5(b) is in proportion to the content of the corresponding acid sites. It is clearly found that the number of acid sits decreases in the used catalysts, which may be the one of reasons for its deactivation in recycling test.
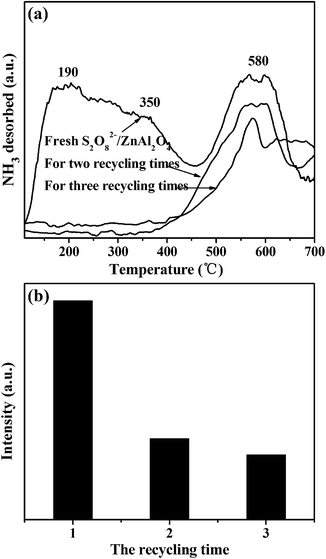 | ||
| Fig. 5 NH3–TPD curves and areas for fresh and used S2O82−/ZnAl2O4-600 (impregnated with 1.50 mol L−1 (NH4)2S2O8 solution). | ||
The near-surface chemical valence states are investigated by XPS within a range of binding energies of 0–1100 eV. Fig. 6 shows the whole XPS spectra of S2O82−/ZnAl2O4-600, revealing the strong peaks of Zn, O, C, S, and Al elements.6 Among them, the observed C element is due to carbon tape from XPS instrument itself. The binding energy of Al 2p at 74.9 eV suggests the presence of Al3+ cation. XPS spectrum of the Zn shows two outstanding peaks are located at 1021.8 eV and 1045.2 eV, corresponding to Zn 2p3/2 and Zn 2p, respectively.22 In addition, the S 2p3/2 peak at 168.95 eV is ascribed to the sulfur oxidation state of +6 which plays an essential role on the formation of the acidity structure.23,24 The suction-induced complex S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O improves the electron-accepting ability of the metal atoms, contributing to the formation of supper acid. This result is consistent with the special bands in the range of 900–1400 cm−1 in IR analysis. The surface sulphur content is 5.97% (atomic ratio), which is the quantitative estimated by means of XPS.
O improves the electron-accepting ability of the metal atoms, contributing to the formation of supper acid. This result is consistent with the special bands in the range of 900–1400 cm−1 in IR analysis. The surface sulphur content is 5.97% (atomic ratio), which is the quantitative estimated by means of XPS.
3.2 Catalytic performance
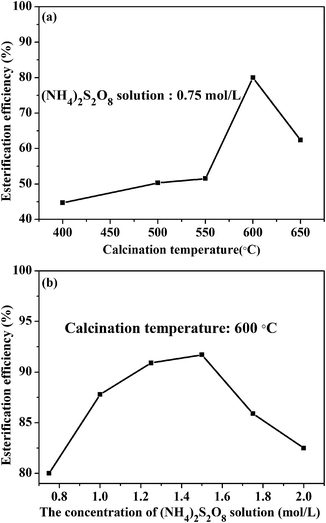
Fig. 7 displays the effects of the calcination temperature and impregnating concentration of the carrier on catalytic activities. These results show that the calcination temperature has a significant influence on catalytic activity. It can be observed that the S2O82−/ZnAl2O4-600 performs the highest catalytic activity with the maximum 80.0% esterification efficiency. Moreover, the results of XRD and FE-SEM show that the S2O82−/ZnAl2O4-600 possesses mesoporous structure and well crystallinity of spinel. These properties may contribute to the high catalytic activity.The influence of impregnating concentration indicates that the catalytic activities are enhanced with increasing the impregnation concentration, reaching a maximum value of 91.7% at 1.5 mol L−1. However, the catalytic activities descend sharply when the concentration further increases. The reason may be that excess sulfate groups covering the surface make the acidity of solid acid decrease.25 Thus an appropriate concentration of 1.5 mol L−1 is favorable for high catalytic activity.
Table 2 shows the effects of reaction conditions on catalytic activities, including the molar ratio, catalyst amount and reaction time. The molar ratio of acetic acid to n-butanol has a significant influence on the esterification efficiency. Stoichiometrically, the molar ratio of acetic acid to n-butanol is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Nevertheless, the excess n-butanol is used to promote the equilibrium to the direction of producing the ester. Accordingly, it is clearly seen that the esterification efficiency of S2O82−/ZnAl2O4-600 increases with increasing the molar ratio of acetic acid to n-butanol from 1
1. Nevertheless, the excess n-butanol is used to promote the equilibrium to the direction of producing the ester. Accordingly, it is clearly seen that the esterification efficiency of S2O82−/ZnAl2O4-600 increases with increasing the molar ratio of acetic acid to n-butanol from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. However, a mass of n-butanol may dilute the concentration of acidity and slow down the reaction.26 Therefore, it witnesses a slight downward trend in the esterification efficiency when the molar ratio exceeds 1
3. However, a mass of n-butanol may dilute the concentration of acidity and slow down the reaction.26 Therefore, it witnesses a slight downward trend in the esterification efficiency when the molar ratio exceeds 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Therefore, the molar ratio of 1
3. Therefore, the molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 is optimum to achieve a high yield.
3 is optimum to achieve a high yield.
| Catalyst | Amountb | Time (h) | a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nc ratio nc ratio |
Esterification efficiency (%) |
|---|---|---|---|---|
| a The synthesis condition: calcination temperature was 600 °C; the concentration of (NH4)2S2O8 solution was 1.50 mol L−1.b The amount was calculated on the basis of total weight of the reactants.c The molar ratio of acetic acid to n-butanol. | ||||
| S2O82−/ZnAl2O4-600a | 1.55 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
56.1 |
| 1.55 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
79.5 | |
| 1.55 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
91.7 | |
| 1.55 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
91.6 | |
| 1.55 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
93.0 | |
| 0.00 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
38.0 | |
| 0.37 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
76.0 | |
| 0.73 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
79.1 | |
| 1.14 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
85.4 | |
| 1.85 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
91.4 | |
| 2.21 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
91.7 | |
| 1.55 | 1.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
77.9 | |
| 1.55 | 2.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
81.3 | |
| 1.55 | 2.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
86.9 | |
| 1.55 | 3.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
91.0 | |
| 1.55 | 4.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
91.4 | |
| ZnAl2O4-600 | 1.55 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
45.2 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| S2O82−/ZnAl2O4-600a catalyst recycling experiments | ||||
| Run 1 | 1.55 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
91.7 |
| Run 2 | 1.55 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
82.6 |
| Run 3 | 1.55 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
72.3 |
| Run 4 | 1.55 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
65.7 |
| Run 5 | 1.55 | 3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
58.9 |
The results of catalytic activities with the various catalyst amounts from 0 to 2.21% are demonstrated in Table 2. The esterification efficiency is 38% when the reaction is carried out without catalyst. The esterification efficiency shows an apparently upward trend with the catalyst amount increasing from 0 to 1.55 wt%. Afterward, the reaction experiences stable esterification efficiency about 91% when the catalyst amount exceeds 1.55 wt%. The reason may be that the excess acid amount might promote the reverse reaction at the mean time.27 Thus, 1.55 wt% is sufficient for the esterification reaction.
The effect of reaction time on the esterification efficiency is shown in Table 2. On the initial stage of 3 h, the esterification efficiency grows rapidly with prolongation of the reaction time. Within 3 h, 91.7% esterification efficiency is achieved. Then, the esterification efficiency is noticed to be stable at around 91% because of the attainment of equilibrium. The optimum reaction time is considered to be 3 h, with the maximum esterification efficiency of 91.7%.
The catalytic activity of ZnAl2O4-600 is performed as the blank experiment. By comparison, it is found that the ZnAl2O4-600 shows lower catalytic activity with 45.16% efficiency. However, the S2O82−/ZnAl2O4-600 catalyst exhibits high catalytic activity with above 90% efficiency.
For the recycling test, the catalyst was filtered after completion of reaction and dried at room temperature without further treatment. Then, the dried catalyst is used for the next recycling experiment with fresh reaction. As shown in Table 2, the catalyst shows an inevitable decrease in catalytic activity after 5 times recycling. The deactivation presumably is owing to the adsorption of organic groups on the surface as well as the reduction of the catalyst amount. Besides, the XRD and FT-IR analyses of the reused catalyst demonstrate that S2O82−/ZnAl2O4-600 has the advantage of structure stability in recycling test.
Table 3 shows the comparison of S2O82−/ZnAl2O4-600 with S2O82−/ZnO-600, S2O82−/Al2O3-600 and other reported solid acid catalysts. The S2O82−/ZnAl2O4-600 catalyst perform the higher catalytic activity than the S2O82−/ZnO-600 and S2O82−/Al2O3-600 catalyst with simple oxide carrier. The catalytic activity of S2O82−/ZnAl2O4-600 is comparable with the series of SO42−/TiO2 and SO42−/ZrO2 catalysts in esterification reactions.28–32 However, crystal structure transformation was commonly observed in the synthesis and modification of SO42−/TiO2 and SO42−/ZrO2, which affected their catalytic performances.3,4 Notably, ZnAl2O4 as a new carrier of solid acid exhibits the advantages of the simpler crystal and excellent crystal structure stability in present work.
| Catalyst | Reaction conditions | Esterification efficiency (%) | Ref. | ||
|---|---|---|---|---|---|
a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) na ratio na ratio |
Amountb (wt%) | Reaction time (h) | |||
| a The molar ratio of acetic acid to n-butanol.b Catalyst amount is calculated on the basis of total weight of the reactants. | |||||
| S2O82−/ZnAl2O4-600 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
1.55 | 3 | 91.7 | Present work |
| S2O82−/ZnO-600 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
1.55 | 3 | 52.9 | Present work |
| S2O82−/Al2O3-600 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
1.55 | 3 | 79.3 | Present work |
| S2O82−/ZrO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
12 | 2 | 91.8 | 28 |
| S2O82−/ZrO2–CeO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
12 | 2 | 96.6 | 28 |
| Sulfated TiO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
12.09 | 2.5 | 92.2 | 29 |
| SO42−–TiO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
2 | 0.75 | 60.1 | 30 |
| SO42−–Ce0.02/TiO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
2 | 0.75 | 97.1 | 30 |
| SO42−/TiO2–Zr–La | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.56 1.56 |
4.88 | 0.5 | 85 | 31 |
| Ti(SO4)2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.69 0.69 |
5.15 | 2 | 90.22 | 32 |
| S-TiO2/MCM-41 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.69 0.69 |
5.15 | 2 | 87.62 | 32 |
Conclusions
A series of S2O82−/ZnAl2O4-T with spinel structure was successfully prepared by sol–gel method. It can be found that the catalytic behaviors were dramatically affected by the calcination temperature. The S2O82−/ZnAl2O4-600 catalyst showed the highest activity. With the molar ratio of acetic acid to n-butyl alcohol of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, the catalyst amount of 1.55 wt% and reaction time of 3 h, the esterification efficiency of S2O82−/ZnAl2O4-600 reached the maximum 91.7%. The characterizations revealed that the ZnAl2O4 carrier had the prominent advantages of single crystal shape and stable structure in the process of sulfidation and recycling test. S2O82−/ZnAl2O4-600 showed mesoporous structures, which may be contributed to high catalytic activity. In conclusion, ZnAl2O4 can be used as a promising carrier for the investigation in solid acid.
3, the catalyst amount of 1.55 wt% and reaction time of 3 h, the esterification efficiency of S2O82−/ZnAl2O4-600 reached the maximum 91.7%. The characterizations revealed that the ZnAl2O4 carrier had the prominent advantages of single crystal shape and stable structure in the process of sulfidation and recycling test. S2O82−/ZnAl2O4-600 showed mesoporous structures, which may be contributed to high catalytic activity. In conclusion, ZnAl2O4 can be used as a promising carrier for the investigation in solid acid.
Acknowledgements
The authors gratefully acknowledge the financial support from NSFC (nos 21203170 and 41472042) and the National College Students' Innovative Training Program (nos 201410491024 and 201310491019), the Fundamental Research Founds for National University (no. 1410491B04) and TLOFCUG (no. SKJ 2013008).Notes and references
- F. Su and Y. H. Guo, Green Chem., 2014, 16, 2934–2957 RSC.
- H. Zhao, P. P. Jiang, Y. M. Dong, M. Huang and B. L. Liu, New J. Chem., 2014, 38, 4541–4548 RSC.
- L. Li, S. W. Liu, J. M. Xu, S. T. Yu, F. S. Liu, C. X. Xie, X. P. Ge and J. Y. Ren, J. Mol. Catal. A: Chem., 2013, 368–369, 24–30 CAS.
- D. H. Guan, M. Q. Fan, J. Wang, Y. Zhang, Q. Liu and X. Y. Jing, Mater. Chem. Phys., 2010, 122, 278–283 CrossRef CAS PubMed.
- G. D. Yadav and R. V. Sharma, J. Catal., 2014, 311, 121–128 CrossRef CAS PubMed.
- Z. R. Zhu, X. Y. Li, Q. D. Zhao, S. M. Liu, X. J. Hu and G. H. Chen, Mater. Lett., 2011, 65, 194–197 CrossRef CAS PubMed.
- T. M. Sankaranarayanan, R. Vijaya Shanthi, K. Thirunavukkarasu, A. Pandurangan and S. Sivasanker, J. Mol. Catal. A: Chem., 2013, 379, 234–242 CrossRef CAS PubMed.
- Z. R. Zhu, Q. D. Zhao, X. Y. Li, H. Li, M. Tade and S. M. Liu, Catal. Sci. Technol., 2013, 3, 788–796 CAS.
- K. Yan, X. Wu, X. An and X. M. Xie, J. Alloys Compd., 2013, 552, 405–408 CrossRef CAS PubMed.
- S. V. Jadhav, K. M. Jinka and H. C. Bajaj, Catal. Today, 2012, 198, 98–105 CrossRef CAS PubMed.
- D. J. Lin, S. F. Shen, H. B. Pan and N. S. Chen, Chin. J. Inorg. Chem., 2000, 16, 757–762 CAS.
- J. X. Wang, H. Pan, D. W. Meng, X. L. Wu, Y. Q. Wang and X. Meng, React. Kinet. Catal. Lett., 2011, 102, 331–341 CrossRef CAS.
- L. Chen, J. X. Wang, D. W. Meng, H. Pan, G. H. Su, Y. Xiao and Z. F. Yan, React. Kinet. Catal. Lett., 2013, 109, 575–587 CrossRef CAS.
- H. Pan, J. X. Wang, L. Chen, G. H. Su, J. M. Cui, D. W. Meng and X. L. Wu, Catal. Commun., 2013, 35, 27–31 CrossRef CAS PubMed.
- K. H. Jiang, D. M. Tong, J. Q. Tang, R. L. Song and C. W. Hu, Appl. Catal., A, 2010, 389, 46–51 CrossRef CAS PubMed.
- R. P. Yao, M. J. Zhang, J. Yang, D. L. Yi, J. Xu, F. Deng, Y. Yue and C. H. Ye, Acta Chim. Sin., 2005, 63, 269–273 CAS.
- S. Farhadi and S. Panahandehjoo, Appl. Catal., A, 2010, 382, 293–302 CrossRef CAS PubMed.
- B. B. Chang, J. Fu, Y. L. Tian and X. P. Dong, Appl. Catal., A, 2012, 437–438, 149–154 CrossRef CAS PubMed.
- V. G. Deshmane and Y. G. Adewuyi, Appl. Catal., A, 2013, 462–463, 196–206 CrossRef CAS PubMed.
- P. F. Chen, M. X. Du, H. Lei, Y. Wang, G. L. Zhang, F. B. Zhang and X. B. Fan, Catal. Commun., 2012, 18, 47–50 CrossRef CAS PubMed.
- C. C. Hwang and C. Y. Mou, Appl. Catal., A, 2009, 365, 173–179 CrossRef CAS PubMed.
- L. J. Liu, G. L. Zhang, L. Wang, T. Huang and L. Qin, Ind. Eng. Chem. Res., 2011, 50, 7219–7227 CrossRef CAS.
- C. W. Dunnill, Z. A. Aiken, A. Kafizas, J. Pratten, M. Wilson, D. J. Morgan and I. P. Parkin, J. Mater. Chem., 2009, 19, 8747–8754 RSC.
- L. G. Devi and R. Kavitha, Mater. Chem. Phys., 2014, 143, 1300–1308 CrossRef CAS PubMed.
- C. H. Li, Y. X. Zhao and B. Dai, J. Ind. Eng. Chem., 2012, 18, 520–525 CrossRef CAS PubMed.
- G. Kuriakose and N. Nagaraju, J. Mol. Catal. A: Chem., 2004, 223, 155–159 CrossRef CAS PubMed.
- T. F. Parangi, B. N. Wani and U. V. Chudasama, Ind. Eng. Chem. Res., 2013, 52, 8969–8977 CrossRef CAS.
- G. D. Fan, M. Shen, Z. Zhang and F. R. Jia, J. Rare Earths, 2009, 27, 437–442 CrossRef.
- H. Zhao, P. P. Jiang, Y. M. Dong, M. Huang and B. L. Liu, New J. Chem., 2014, 38, 4541–4548 RSC.
- G. Xiao, J. F. Zhou, X. Huang, X. P. Liao and B. Shi, RSC Adv., 2014, 4, 4010–4019 RSC.
- W. P. Shi, Catal. Lett., 2013, 143, 732–738 CrossRef CAS PubMed.
- Y. H. Wang, Y. T. Gan, R. Whiting and G. Z. Lu, J. Solid State Chem., 2009, 182, 2530–2534 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |