Freestanding 3D graphene/cobalt sulfide composites for supercapacitors and hydrogen evolution reaction†
Abstract
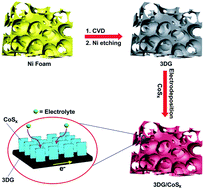
The development of lightweight, flexible, electrochemically active materials with high efficiency is important for energy storage and conversion. In this study, we report the fabrication of a freestanding, 3-dimensional graphene/cobalt sulfide nanoflake (3DG/CoSx) composite for supercapacitors and hydrogen evolution catalysts. The graphene framework formed by chemical vapour deposition provides superlight, highly conductive electron transport pathways, as well as abundant pores for electrolyte penetration. The densely patterned cobalt sulfide nanoflake arrays grown by electrodeposition offer a large surface area for electrochemical reactions, high theoretical capacitance and efficient hydrogen evolution catalytic activity. As a proof-of-concept, supercapacitors made of the 3DG/CoSx composites deliver a high specific capacitance of 443 F g−1 at 1 A g−1, with excellent capacity retention of 86% after 5000 cycles and mechanical flexibility. In addition, the 3DS/CoSx composites show attractive features as hydrogen evolution catalysts, with a low overpotential of 0.11 V and a Tafel slope of 93 mV dec−1.

 Please wait while we load your content...
Please wait while we load your content...