Stark sublevels in Tm3+–Yb3+ codoped Na2Y2B2O7 nanophosphor for multifunctional applications
Abstract
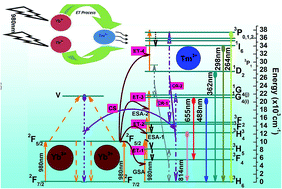
The phase and crystal structure of the Na2Y2B2O7:Tm3+–Yb3+ inorganic phosphor prepared by solution combustion method have been identified by powder X-ray diffraction technique. Surface morphology and particle size have been examined using field emission scanning electron microscopy and high resolution transmission electron microscopy characterizations of the prepared materials. No absorption band around 980 nm has been observed in the Tm3+ doped phosphors, whereas a broad band around 980 nm in the Tm3+–Yb3+ codoped phosphors corresponding to the 2F7/2 ← 2F5/2 absorption transition of Yb3+ ion has been detected. Upconversion emission bands have been observed in the UV, visible and NIR regions upon excitation with a 980 nm laser diode. The temperature sensing behaviour and the concept of a nanoheater of the developed nanophosphor have been demonstrated by using the stark sublevels of the 1G4 level of the Tm3+ ion, which are responsible for the blue upconversion emission. A maximum sensor sensitivity of about 4.54 × 10−3 K−1 at 300 K for the developed multifunctional nanophosphor has been determined. A temperature gain of about ∼435 K has been observed at a laser power density of 66.88 W cm−2 and the colour coordinates do not change with the variation of the pump power density. For localizing and heating hyperthermia based cancer cells using NIR radiation, a very low pump power density of about ∼7.0 W cm−2 has been established. The experimental observations prove that the developed material can be used as a multifunctional nanomaterial in optical devices and biological applications.

 Please wait while we load your content...
Please wait while we load your content...