Superparamagnetic Fe3O4 nanoparticles modified by water-soluble and biocompatible polyethylenimine for lipase immobilization with physical and chemical mechanisms†
Weiwei Zhu‡
,
Yijing Li‡,
Fang Zeng,
Hang Yin,
Liyuan Wang and
Hao Zhu*
State Key Laboratory of Applied Organic Chemistry, Key Laboratory of Nonferrous Metal Chemistry and Resources Utilization of Gansu Province, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000, China. E-mail: zhuhao07@lzu.edu.cn; Fax: +86-931-8912113; Tel: +86-931-8912528
First published on 19th February 2015
Abstract
Superparamagnetic Fe3O4 nanoparticles with diameter about 18 nm were prepared by a solvothermal method. A simple and green method was used to prepare amino-rich magnetic nanoparticles. The magnetic nanoparticles were modified by polyethylenimine (PEI) which is a polycationic polymer when pH < 10. The resulted magnetic nanoparticles were activated by glutaraldehyde to obtain nanoscale support for Candida rugosa Lipase (CRL) immobilization. The CRL was immobilized on the magnetic nanoparticles covalently, as well as via ionic exchange. The structure and magnetic behavior of the magnetic nanoparticles were confirmed by transmission electron microscopy (TEM), Fourier transform infrared spectroscopy (FT-IR), and vibrating sample magnetometer (VSM). Then the properties of the immobilized CRL were investigated, and the results showed that the obtained immobilized lipase displayed good reusability and applicability. The immobilized Candida rugosa Lipase (ICRL) presented wider pH tolerance (residual activity > 80% in a pH range from 5.5 to 8.0) than free Candida rugosa Lipase (residual activity decreased rapidly when the pH values were away from 7.0). ICRL showed excellent thermal stability (the relative of ICRL > 90%) after keeping in the water bath at 50 °C for 150 min while free Candida rugosa Lipase (FCRL) was absolutely deactivated. After being reused 10 times, ICRL maintained 60% relative activity.
1. Introduction
As one of the most basic and important magnetic materials, Fe3O4 has attracted much attention in recent years because of its wide-ranging applications for electronic devices, catalysis, biomedical utilizations, drug delivery, enzyme immobilization and so on.1–7 With further studies, Fe3O4 nanoparticles display different properties during its applications with different modified polymers such as polyethylenimine, poly(methyl methacrylate), poly(maleic monoester) and polyaniline.8–13 PEI is a water-soluble and biocompatible polyamine with amino-rich macromolecular chains. PEI has primary, secondary and tertiary amino groups, with the possibility of reaction with different compounds.14 Gene vector, gas adsorption and heavy metal ions adsorption are some applied fields of PEI.15–19 Otherwise, the amino groups of PEI are protonated and have positive charges when the pH < 10. Chen et al.20 prepared a new resin by alkylation of branched PEI beads which can selectively extract trace amounts of ClO4− from a makeup groundwater in the presence of competing ions. Zhang et al.21 utilized PEI functions as a co-catalyst for CO2 reduction to formate in aqueous media without metal catalyst, which significantly reduced catalytic overpotential and increased current density and efficiency. Lou et al.22 reported that PEI was self-assembled on the surface of Fe3O4 nanoparticles to immobilize Quantum dots.In contrast with chemical catalyst, enzyme expressed excellent catalytic properties: high effectiveness, high specificity, and mild reaction conditions.23 Among the lipases from various sources, Candida rugosa Lipase has drawn much attention because of its high activity and broad specificity.24 However, the industrial applications of enzyme exhibits numerous problems such as high operation cost, low stability, difficult recyclability and reusability.25 Immobilized enzyme, especially immobilized on magnetic supports which have many advantages including large specific surface area, easily dispersion, catalytic stability, operational stability, easy recycling and reusing, vast reduction of costs etc., provides good solutions to those problems.23,26–28 Recently, nanomaterials have emerged as promising supports for enzymes immobilization.29–31 Also, superparamagnetic Fe3O4 nanoparticles as an interesting supports for lipase immobilization have drawn the attention of numerous workers in recent years.32–36
Besides immobilization materials, the immobilization methods also play an important role in the activity of immobilized enzyme.37 Covalent binding,38 entrapment39 and adsorption40 are common strategies to immobilize enzyme. Adsorption doesn't chemically modify the lipase and is relatively simple method of immobilization,41 but it is not strong enough to prevent the enzyme leakage and it may lead to low specificity of the reaction.42 Covalent binding is a stronger method to immobilize enzyme which can prevent the enzyme leaching from the support efficiently.41 Enzyme immobilization may provide an improved enzyme performance. According to the reports, enzyme stability may be obviously improved if an intense multipoint covalent attachment via a short spacer arm between an enzyme molecule and a rigid support is achieved or if a favorable nano-environment is achieved.43 Moreover, immobilized enzymes in some instances possess enhanced specificity, selectivity storage and operational stability towards multifarious denaturing agents and possibly restraint inhibition.42 But some drawbacks of covalent binding still exist, such as possible decrease of enzymatic activity, possibility sterical modifications of the enzyme, chemical modifications of the support are necessary and usually irreversible attachment, preventing support reuse.42 In fact, it has been proved that immobilization with multifarious methods would greatly increase the loading amounts of enzyme and improve the stability of enzyme.44 So the supports with both cationic groups and chemically reactive groups (for instance, aldehyde group, epoxy group) would be a sort of promising supports.
In some reports, PEI was used to modify magnetic supports and immobilize enzyme with adsorption.45,46 In this paper, we prepared a kind of superparamagnetic Fe3O4 nanoparticles modified by PEI with a simple, green and economic method. The nanoparticles were activated by glutaraldehyde to form nanoscale supports for lipase immobilization with covalent connection and ionic exchange respectively. The stability and properties of immobilized Candida rugosa Lipase were researched. And the results showed that the supports we prepared were good enough to immobilize lipase.
2. Materials and methods
2.1 Materials
Iron(III) chloride hexahydrate (FeCl3·6H2O), diethylene glycol are analytical grade and purchased from Tianjing Guangfu Fine Chemical Industry Research Institute (China). Sodium citrate and sodium acetate are chemical grade and purchased from Tianjing Guangfu Fine Chemical Industry Research Institute (China). PEI (Mw = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was obtained from Aladdin Industrial Co. (Shanghai, China). CRL (Type VII, 1180 U mg−1 solid) and bovine serum albumin (BSA) were purchased from Sigma Chemical Co. Other chemicals and solvents were all of analytical grade and obtained from Tianjing Chemical Reagent Company (China).
000) was obtained from Aladdin Industrial Co. (Shanghai, China). CRL (Type VII, 1180 U mg−1 solid) and bovine serum albumin (BSA) were purchased from Sigma Chemical Co. Other chemicals and solvents were all of analytical grade and obtained from Tianjing Chemical Reagent Company (China).
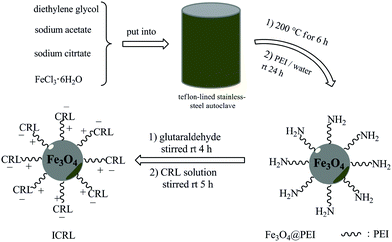
2.2 Preparation and functionalization of superparamagnetic Fe3O4@PEI nanospheres
2.3 Characterization of magnetic support
The morphologies of Fe3O4, Fe3O4@PEI were examined by transmission electron microscopy (TEM, FEI Tecnai G20). The crystal structure of Fe3O4 and Fe3O4@PEI was observed by X-ray diffraction (XRD, Rigaku D/MAX-2400 X-ray diffractometer with Ni-filtered Cu Kα radiation). The structures of samples were obtained in transmission mode on a Fourier-transform infrared spectrophotometer (FT-IR, American Nicolet Corp. Model 170-SX) using the KBr pellet technique. The magnetization curves of Fe3O4 and Fe3O4@PEI were confirmed by transmission electron microscopy (TEM, LAKESHORE-7304, USA) at room temperature. At a heating rate of 10.0 K min−1, Thermogravimetric (TG) analysis of Fe3O4 and Fe3O4@PEI was observed by a TGDSC apparatus (NETZSCH STA 449C) by heating the samples from room temperature to 800 °C under a N2 atmosphere.2.4 Immobilization of CRL
The supports were activated with both cationic groups and chemically reactive groups, so lipase could be immobilized on this support by ionic exchange and covalent bonding concurrently. On the basis of the reaction between aldehyde group and amino group of lipase,49 the lipase immobilization was taken place by reaction between lipase solution and magnetic supports directly. Necessary qualities of supports were added into CRL solution (m/v, 1%), then the lipase immobilization was carried out under the conditions of magnetic stirring at room temperature for 5 h. After the reaction completed, ICRL was obtained by magnetic separation and washed with phosphate buffer (0.1 M, pH 7.0) several times to remove the noncovalently coupled lipase. The resulted ICRL was kept at 4 °C for further utilization. In addition, the reaction solution and washing solution were collected to assay the amount of residual lipase. Finally, the influences of pH value, temperature and the amount of lipase added on the activity of ICRL were investigated.2.5 Determination of immobilization efficiency and lipase activity
The immobilization efficiency was expressed by the amounts of enzyme bounded on supports of unite mass, and the amount of enzyme was determined by the Bradford method,50 using BSA as the standard. The enzymatic activities of free and immobilized lipase were measured by the titration of the fatty acid which comes from the hydrolysis of olive oil51 and reverse titration. One unit of lipase activity (U) is defined as the amount of enzyme needed to hydrolyze olive oil liberating 1.0 μmol of fatty acid per min in the assay condition. Herein, the efficiency of immobilization was evaluated in terms of activity yields and immobilization yield as follows:| Activity yield (%) = (C/A) × 100% |
| Immobilization yield (%) = [(A − B)/A] × 100% |
All data used in these formulas are the average of triplicate of experiments.
2.6 Properties of ICRL
3. Results and discussion
3.1 Preparation and characterization of magnetic nanospheres
D = kλ/(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) θ) |
The TEM images of Fe3O4 nanoparticles and Fe3O4@PEI are shown in Fig. 2. It can be seen in Fig. 2a that the Fe3O4 particles are spherical and the dimension of the particles is of nanometer grade. The average diameter of Fe3O4 nanoparticles are about 18 nm according to Fig. 2. Besides, the primary Fe3O4 nanoparticles displayed unsatisfactory dispersity because of the interface effects of nanoparticles and the plentiful hydroxyl on the surface of nanoparticles. The morphologies of Fe3O4@PEI are shown in Fig. 2b. After being decorated by PEI, the diameter of nanoparticle is almost the same, while the dispersity of nanoparticle shows great improvement obviously.
Fig. 3 gives the FT-IR spectra of (a) Fe3O4 and (b) Fe3O4@PEI. Both in Fig. 3a and b, there were adsorption peaks presented at 584 cm−1 belong to the characteristic absorption of Fe–O bonds in Fe3O4. In addition, the intensity of Fe3O4 vibration peaks of (584 cm−1) in Fig. 3b became weaker than that in Fig. 3a, because Fe3O4 was modified by PEI. The symmetric and asymmetric stretching vibrations of carboxylate could be found at 1616 cm−1 and 1396 cm−1. In contrast with Fig. 3a, new adsorption peaks appeared at 1263 cm−1 and 1662 cm−1 in Fig. 3b were the stretching vibration absorption of –NH2. From the data above, it could be indicated that the surface of Fe3O4 was modified by a number of amino groups, i.e. Fe3O4 was modified by PEI successfully.54
The magnetic properties of the magnetic nanoparticles were confirmed by VSM at room temperature. As shown in Fig. 4, the saturation magnetizations of pure Fe3O4 and Fe3O4@FEI were 78.5 and 58.2 emu g−1, respectively. It can be estimated that the percentage of PEI in the resulted Fe3O4@PEI was 25.9%. Because of the large saturation magnetization, these supports can be easily and rapidly separated from the reaction system. Moreover, there was no hysteresis in the magnetization with both remanence and coercivity,55 which can prove that these magnetic supports are superparamagnetic. Therefore, these magnetic supports could respond to an applied magnetic field without any permanent magnetization and be redispersed rapidly when the magnetic field disappeared.
Fig. 5 shows the TG analysis of nanospheres Fe3O4 and Fe3O4@PEI. At a heating rate of 10.0 K min−1, TG analysis of Fe3O4 (a) and Fe3O4@PEI (b) was observed by heating the samples from room temperature to 800 °C under a N2 atmosphere. As given in Fig. 5a, a weight loss of 8.12% was found when temperature rose to 800 °C. The weight loss was probably due to removal of citrate on the surface of nanospheres. Compared with Fig. 5a, an additional weight loss about 11.9% was observed in Fig. 5b. This also proved Fe3O4 nanoparticles to be decorated with PEI successfully.
3.2 Optimum lipase amounts added of CRL immobilization
The influence of lipase amounts added on the activities of ICRL was studied (Fig. s-1†). Immobilization reaction was tested in phosphate solution (0.1 M, pH = 7.0) at 30 °C for 5 h. The relative activities of ICRL increased obviously with the increasing amount of lipase until the relative activity of ICRL reached a maximum value, at which the amount of protein given was 250 mg g−1 support. After this, the relative activities of ICRL gradually decreased. The same phenomenon has been reported in our previous work.56 The reasons for this phenomenon might be considered as (1) the competition between CRL and other proteins of the extract by the surface of the support. For the lipase solution contained not only CRL but also other protein, when CRL immobilized on supports, other protein connected to the supports as well. Hence, the amounts of immobilized CRL were restricted; (2) an intermolecular steric hindrance was formed with higher enzyme loading, which caused decrease of enzymatic activity.3.3 Properties of ICRL
3.3.2.1 Effect of denaturant on the activity of ICRL. Carbamide was chosen as denaturant to research effect of denaturant on the activity on ICRL. The relationship between the activity of lipase and the amounts added of denaturant is shown in Fig. 6. The relative activities of both FCRL and ICRL decreased directly with the increasing amount of urea. FCRL retrained about 30% of relative activity when the concentration of urea was 3.0 mol L−1; ICRL still retained 70% of relative activity. FCRL was deactivated absolutely when the concentration of urea was increased to 6.0 mol L−1; ICRL remained 55% of relative activity. The reason for the tolerance enhancement of denaturant of ICRL might because the reactive probabilities between lipase and denaturant was decreased when lipase was immobilized on supports.
3.3.2.2 The thermal stability of ICRL. The stability of ICRL and FCRL were measured by the method mentioned in our experiment section and the results were shown in Fig. 7. In the picture, FCRL was absolutely deactivated after keeping in the water bath for 150 min, while ICRL presented excellent stability (the relative of ICRL > 90%). The excellent performance of ICRL was maintained even if it was kept in water at 50 °C after 210 minutes. It can be concluded from the figure that ICRL displayed better thermal tolerance than FCRL.
3.3.2.3 Reusability of ICRL. Reusability of ICRL is very important. It could save resource and reduce the industrial cost effectively. In fact, the reusability of ICRL is a very significant index to evaluate the pros and cons of properties of supports. The activity of ICRL was slowly decreased after several cycles. After being reused 10 times, the relative activity of ICRL still maintained 60% (Fig. s-4(a)†). The reduction of enzymatic activity perhaps contains (1) not all of the particles were captured and (2) some lipase ran off from the supports during reusing of ICRL. In addition, CRL which was immobilized on the supports with only ionic exchange showed poor reusability after being reused 5 times (the relative activity < 10%, Fig. s-4(b)†). This might because the physical bond is not strong enough to prevent most lipases leaching from the supports during reusing.41 It could be summarized a conclusion from the experimental data that ICRL with chemical and physical method exhibited better operability and stability than that ICRL with only ionic exchange.
4. Conclusions
In conclusion, superparamagnetic nanoparticles were prepared with a solvothermal method, and modified by PEI to obtain nanoscale magnetic supports for CRL immobilization. CRL was immobilized on the supports with high-efficiency for the immobilization strategies included both chemical action and physical process. The optimal conditions for lipase immobilization were further studied. Overall, though the activity of ICRL is lower than FCRL in the same catalytic conditions, the ICRL we prepared had higher operational stability and exhibited excellent properties, for example, pH endurance (ICRL displayed good activity in a wider pH range from 5.5 to 8.0 than FCRL), temperature endurance (high activity of ICRL was observed when the temperature elevated from 35 °C to 55 °C), and excellent reusability (ICRL was reused for 10 times without any significant decrease of catalytic activities). In a word, the process we introduced in this paper is recommendable for lipase immobilization.Acknowledgements
The authors thank the financial supports from the National Natural Science Foundation of China (no. 21374045, no. 21074049). This paper is dedicated to the memory of pro. Yanfeng Li, who passed away on 31st Dec. 2014.Notes and references
- S. F. Wang, F. Xie and R. F. Hu, Sens. Actuators, B, 2007, 123, 495–500 CrossRef CAS.
- O. A. Aktsipetrov, Colloids Surf., A, 2002, 202, 165–173 CrossRef CAS.
- P. Martins, C. M. Costa and S. Lanceros-Mendez, Appl. Phys. A, 2011, 103, 233–237 CrossRef CAS.
- L. Pust, L. E. Wenger, R. A. Lukaszew, Y. Sheng, D. Litvinov, Y. Wang, C. Uher and R. Clarke, J. Appl. Phys., 1999, 85, 5765–5767 CrossRef CAS.
- M. A. McHugha, F. Rindfleischa, P. T. Kuntza, C. Schmaltzb and M. Buback, Polymer, 1998, 39, 6049–6052 CrossRef.
- N. Kato, Y. Takizawa and F. Takahashi, J. Intell. Mater. Syst. Struct., 1997, 8, 588–595 CrossRef CAS.
- B. R. White, B. T. Stackhouse and J. A. Holcombe, J. Hazard. Mater., 2009, 161, 848–853 CrossRef CAS PubMed.
- J. Jin, F. Yang, F. Zhang, W. Q. Hu, S. B. Sun and J. T. Ma, Nanoscale, 2012, 4, 733–736 RSC.
- H. Chen, C. Deng and X. Zhang, Angew. Chem., Int. Ed., 2010, 49, 607–611 CrossRef CAS PubMed.
- D. K. Lee, Y. S. Kang, C. S. Lee and P. Stroeve, J. Phys. Chem. B, 2002, 106, 7267–7271 CrossRef CAS.
- J. Deng, C. He, Y. Peng, J. Wang, X. Long, P. Li and A. S. Chan, Synth. Met., 2003, 139, 295–301 CrossRef CAS.
- S. Xuan, Y. X. J. Wang, J. C. Yu and K. C. F. Leung, Langmuir, 2009, 25, 11835–11843 CrossRef CAS PubMed.
- K. P. Lee, A. Gopala, S. Komathi and D. Raghupathy, Phys. Prop. Appl. Polym. Nanocompos., 2010, 187 CAS.
- R. Bahulekar, N. R. Ayyangar and S. Ponrathnam, Enzyme Microb. Technol., 1991, 13(11), 858–868 CrossRef CAS PubMed.
- J. K. Towns and F. E. Regnier, J. Chromatogr. A, 1990, 516, 69–78 CrossRef CAS PubMed.
- J. W. Schurer, D. Kalicharan, P. J. Hoedemaeker and I. Molenaar, J. Histochem. Cytochem., 1978, 26, 688–689 CrossRef CAS PubMed.
- R. R. Navarro, K. Sumi, N. Fujii and M. Matsumura, Water Res., 1996, 30, 2488–2494 CrossRef CAS.
- T. Xia, M. Kovochich, M. Liong, H. Meng, S. Kabehie, S. George and A. E. Nel, ACS Nano, 2009, 3, 3273–3286 CrossRef CAS PubMed.
- A. Manjon, J. L. Iborra and A. Arocas, Biotechnol. Lett., 1991, 13, 339–344 CrossRef CAS.
- D. P. Chen, C. Yu, C. Y. Chang, Y. Wan, J. M. J. Frechet, W. A. Goddard III and M. S. Diallo, Environ. Sci. Technol., 2012, 46, 10718–10726 CrossRef CAS PubMed.
- S. Zhang, P. Kang, S. Ubnoske, M. K. Brennaman, N. Song, R. L. House and T. J. Meyer, J. Am. Chem. Soc., 2014, 136, 7845–7848 CrossRef CAS PubMed.
- L. Lou, K. Yu, Z. Zhang, B. Li, J. Zhu, Y. Wang and Z. Zhu, Nanoscale, 2011, 3, 2315–2323 RSC.
- J. Hong, D. M. Xu, P. J. Gong, H. W. Sun, L. Dong and S. J. Yao, J. Mol. Catal. B: Enzym., 2007, 45, 84–90 CrossRef CAS.
- P. D. Marıa, J. M. S. Montero, J. V. Sinisterra and A. R. Alcantara, Biotechnol. Adv., 2006, 24, 180–196 CrossRef PubMed.
- S. W. Chang, J. F. Shaw, K. H. Yang, S. F. Chang and C. J. Shieh, Bioresour. Technol., 2008, 99, 2800–2850 CrossRef CAS PubMed.
- S. Pahujani, S. Kanwar, G. Chauhan and R. Gupta, Bioresour. Technol., 2008, 99, 2566–2570 CrossRef CAS PubMed.
- D. S. Jiang, S. Y. Long, J. Huang, H. Y. Xiao and J. Y. Zhou, Biochem. Eng. J., 2005, 25, 15–23 CrossRef CAS.
- R. C. Rodrigues, C. Ortiz, Á. Berenguer-Murcia, R. Torres and R. Fernández-Lafuente, Chem. Soc. Rev., 2013, 42(15), 6290–6307 RSC.
- A. A. Vertegl, R. W. Siegal and J. S. Dordick, Langmuir, 2004, 20, 6800–6807 CrossRef PubMed.
- P. Wang, Curr. Opin. Biotechnol., 2006, 17, 574–579 CrossRef CAS PubMed.
- M. N. Gupta, M. Kaloti, M. Kapoor and K. Solanki, Artif. Cells, Blood Substitutes, Biotechnol., 2011, 39, 98–109 CrossRef CAS PubMed.
- D. K. Kim, M. Mikhaylova, Y. Zhang and M. Muhammed, Chem. Mater., 2003, 15, 1617–1627 CrossRef CAS.
- C.-L. Chiang, C.-S. Sung, T.-F. Wu, C.-Y. Chen and C.-Y. Hsu, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2005, 822, 54–60 CrossRef CAS PubMed.
- J. Kim, J. W. Grate and P. Wang, Trends Biotechnol., 2008, 26, 639–646 CrossRef CAS PubMed.
- C. G. C. M. Netto, L. H. Andrade and H. E. Toma, Tetrahedron: Asymmetry, 2009, 20, 2299–2304 CrossRef CAS.
- G. Zhao, J. Wang, Y. Li, X. Chen and Y. Liu, J. Phys. Chem. C, 2011, 115(14), 6350–6359 CAS.
- Y. Omprakash and I. Toyoko, Biomacromolecules, 2005, 62, 809–2814 Search PubMed.
- J. Hong, P. J. Gong, J. H. Yu, D. M. Xu, H. W. Sun and S. J. Yao, J. Mol. Catal. B: Enzym., 2006, 42, 99–105 CrossRef CAS.
- Y. J. Wang and F. Caruso, Chem. Mater., 2005, 17, 953–961 CrossRef CAS.
- S. Koutsopoulos, J. Oost and W. Norde, Langmuir, 2004, 20, 6401–6406 CrossRef CAS PubMed.
- D. Brady and J. Jordaan, Biotechnol. Lett., 2009, 31(11), 1639–1650 CrossRef CAS PubMed.
- P. Zucca and E. Sanjust, Molecules, 2014, 19, 14139–14194 CrossRef PubMed.
- O. Barbosa, C. Ortiz, A. Berenguer-Murcia, R. Torres, R. C. Rodrigues and R. Fernandez-Lafuente, RSC Adv., 2014, 4, 1583–1600 RSC.
- B. C. C. Pessela, C. Mateo, A. V. Carrascosa, A. nVian, J. L. Garcia, G. Rivas, C. Alfonso, J. M. Guisan and R. F. Lafuente, Biomacromolecules, 2003, 4, 107–113 CrossRef CAS PubMed.
- K. Solanki and M. N. Gupta, New J. Chem., 2011, 35(11), 2551–2556 RSC.
- J. Mukherjee, K. Solanki and M. N. Gupta, Methods Mol. Biol., 2013, 1051, 117–127 CAS.
- L. Shen, J. Bao, D. Wang, Y. X. Wang, Z. W. Chen, L. Ren and A. Q. Yang, Nanoscale, 2013, 5, 2133–2141 RSC.
- P. Monsan, J. Mol. Catal., 1978, 3(5), 371–384 CrossRef CAS.
- O. Barbosa, R. Torres, C. Ortiz, Á. Berenguer-Murcia, R. C. Rodrigues and R. Fernandez-Lafuente, Biomacromolecules, 2013, 14(8), 2433–2462 CrossRef CAS PubMed.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS PubMed.
- N. Watanabe, Y. Ota, Y. Minoda and K. Yamada, Agric. Biol. Chem., 1977, 41, 1353–1358 CrossRef CAS.
- I. Migneault, C. Dartiguenave, M. J. Bertrand and K. C. Waldron, BioTechniques, 2004, 37(5), 790–806 CAS.
- C. Garcia-Galan, A. Berenguer-Murcia, R. Fernandez-Lafuente and R. C. Rodrigues, Adv. Synth. Catal., 2011, 353, 2885–2904 CrossRef CAS.
- Y. Wang, F. Xu, L. Zhang and X. Wei, J. Nanopart. Res., 2013, 15, 1–11 Search PubMed.
- Z. Ma, Y. Guan and H. Liu, J. Magn. Magn. Mater., 2006, 301, 469–477 CrossRef CAS.
- X. Liu, X. Chen, Y. Li, X. Wang, X. Peng and W. Zhu, ACS Appl. Mater. Interfaces, 2012, 4, 5169–5178 CAS.
- D. Brady and J. Jordaan, Biotechnol. Lett., 2009, 31(11), 1639–1650 CrossRef CAS PubMed.
- (a) H. Demircioğlu, H. Beyenal, A. Tanyolaç and N. Hasirci, Polym. Int., 1994, 35, 321–327 CrossRef; (b) C. Mateo, J. M. Palomo, G. Fernandez-Lorente, J. M. Guisan and R. Fernandez-Lafuente, Enzyme Microb. Technol., 2007, 40, 1451–1463 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra15832f |
| ‡ These authors contributed equal work. |
| This journal is © The Royal Society of Chemistry 2015 |