Stereoselective ring-opening polymerization of rac-lactides catalyzed by titanium complexes containing N,N-bidentate phenanthrene derivatives†
Bo Gao*ab,
Qian Duana,
Yanhui Lia,
Dongni Liac,
Liqiu Zhanga,
Yuan Cui*a,
Ninghai Hub and
Xuan Pangb
aSchool of Materials Science and Engineering, Changchun University of Science and Technology, Changchun 130022, China. E-mail: gaobo@ciac.ac.cn; cuiyuancust@163.com; Fax: +86-431-85583016; Tel: +86-431-85583016
bKey Laboratory of Polymer Ecomaterials, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022, China
cDepartment of Blood Transfusion, China–Japan Union Hospital, Jilin University, Changchun, 130033, China
First published on 14th January 2015
Abstract
Three titanium complexes with N,N-bidentate phenanthrene derivatives (1: R = iPr, 2: R = C6H5, 3: R = 2,6-iPr2C6H3) were synthesized. These complexes were characterized using 1H/13C NMR spectroscopy and elemental analysis and employed in the ring-opening polymerization (ROP) of L-lactide and rac-lactide. Complex 1 displayed the highest activity among these complexes for ROP of L-lactide, and complex 3 showed the highest stereoselectivity for the ROP of rac-lactide attaining a partially heterotactic polylactide (PLA) with a Pr of 0.63. The polymerization kinetics employing 3 as the catalyst were researched. The kinetic studies revealed that the polymerization rate was first-order with respect to the monomer.
Introduction
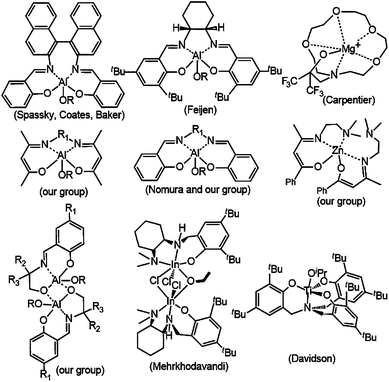
Nowadays, polylactide (PLA), derived from lactic acid, is obtained from renewable resources such as corn and sugarbeet.1,2 It is recognized as one of the most promising environmentally-friendly polyesters exhibiting biological degradation.2–4 The presence of two chiral centers in the lactide (LA) monomer results in different lactide stereoisomers, namely L-lactide (L-LA), D-lactide (D-LA) and meso-lactide (Fig. 1). The stereochemistry of the polymer chains influences the PLA’s physical and chemical properties. In general, PLA is synthesized via ring-opening polymerization (ROP) of lactide that is initiated using metal complexes, such as Sn,5,6 Al,7–9,22,23 Zn,10–12 Mg,13–15 Ti,16,17 In,18 and rare-earth metal complexes.19–21 Among them, some titanium complexes were proven to be efficient in the ROP of lactide. It has been reported that the activities of titanium complexes in lactide polymerization were obviously influenced by the identity of the metal and the ligand.16,17 In the past twenty years, many efforts have been made for the selection of proper ancillary ligands for enhancing the performance of the initiators in polymerizations. A number of researchers tried to expound the relation among rac-lactide (rac-LA), metal complexes and the tacticity of polymers.22,23 In recent years, our lab has studied many aluminum and zinc metal complexes based on nitrogen-based ligands (see Fig. 2).23 These complexes were proven to be very efficient initiators in the ROP of lactide.Recently, we have become curious about the catalytic behaviour of titanium complexes containing N,N-bidentate phenanthrene derivatives. As far as we know, few studies on titanium complexes bearing this type of ligand have been investigated for the stereoselective ROP of rac-LA (Fig. 3).
Based on the efficient use of titanium complexes,16,17 we speculate that this type of titanium complex bearing N,N-bidentate diamido ligands might be a potential catalyst for the ROP of rac-LA. In this work, we report the preparation of a number of titanium complexes bearing phenanthrene derivatives and their activities toward the ROP of lactide.
Experimental
General
All experimental operations were performed using Schlenk line techniques. Elemental analysis was accomplished using a Varian EL microanalyzer; 1H/13C NMR spectra were recorded on a Bruker 300 MHz or 400 MHz spectrometer using CDCl3 solvent for compounds and polymers. Gel permeation chromatography measurements were performed on a Waters 515 GPC with CHCl3 as the eluent (flow rate: 1 mL min−1, at 35 °C). The molecular weight was adjusted through a PS standard. Crystallographic data were gathered and analyzed according to the experiments in ref. 23a and 34. 2,2′-Biphenyldicarboxaldehyde, isopropylamine, aniline, 2,6-diisopropyl aniline, ytterbium(III) trifluoromethanesulfonate, samarium(II) iodide and dichlorotitanium diisopropoxide were purchased from the J&K Scientific company.Synthesis of pro-ligands
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Vethyl acetate = 10
Vethyl acetate = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in 52.5–63.4% yield.
1) in 52.5–63.4% yield.Synthesis of titanium complexes
General procedures for lactide polymerization
Result and discussion
Synthesis of pro-ligands and titanium complexes
As shown in Fig. 3, the pro-ligands L1–L3 were synthesized easily via condensation reaction between 2,2′-biphenyldicarboxaldehyde and a modified amine, and then through an intermolecular coupling reaction catalyzed by 4 equivalents of SmI2 with 2 equivalents of Yb(OTf)3, in a process similar to the one of compound 20a in ref. 24, in moderate yields (52.5–63.4%).Titanium complexes 1–3 were synthesized via a one-pot synthesis. The lithiation of the pro-ligands L1–L3 with 2 equiv. of n-BuLi produced the corresponding lithium salts. The lithiation reaction was performed at −78 °C to restrict the generation of byproducts because this kind of reaction is generally rapid and exothermic. Then, the reactions of TiCl2(OiPr)2 with the lithium salts were carried out at −20 °C, and titanium complexes 1–3 were attained as red solids in good yields (79.3–85.7%). All three complexes were sensitive to moisture.
Complexes 1–3 were characterized using 1H/13C NMR in CDCl3 and elemental analysis. The 1H and 13C NMR spectra of complexes 1–3 showed one ligand and two isopropoxide groups coordinated to the titanium atom. They showed similar resonances in the high field at δ = 4.88–3.47 ppm, which were attributed to the two secondary protons of the –OCH(CH3)2 groups, and at δ = 1.19–0.77 and 0.92–0.62 ppm which were assigned to the primary protons of the –OCH(CH3)2 groups in the 1H NMR spectra. And the disappearance of the signals at δ = 4.72–3.25 ppm, in contrast to the pro-ligands, was attributed to the diamide –RNH– protons, which suggested that the nitrogen atoms of the N,N-bidentate ligands were coordinated to the Ti atoms. The results were consistent with the single crystal structure of 3 in the solid state. The geometry of complex 3 in the solid state was confirmed via X-ray diffraction analysis. The molecular structure is depicted in Fig. 4. The selected bond distances and angles and other crystallographic data are depicted in Tables S1 and S2 (see ESI†), respectively. X-ray structural analysis revealed that complex 3 was mononuclear and the central titanium atom was coordinated by the two N atoms from the N,N-bidentate ligand and two O atoms from the isopropoxy groups. In complex 3, the Ti1–N2 bond length, 1.919(4) Å, is the longest among the Ti–X bond lengths (Ti1–N1: 1.893(4) Å, Ti1–O1: 1.798(4) Å, Ti1–O2: 1.856(4) Å, which were in the normal range found in related titanium complexes25–27); the O1–Ti1–O2, O1–Ti1–N1, O2–Ti1–N1, O1–Ti1–N2, O2–Ti1–N2 and N1–Ti1–N2 angles are 107.53(7), 116.34(7), 109.62(8), 116.34(7), 117.23(7) and 89.09(6)°, respectively. Obviously, the titanium atom was in a distorted tetrahedral geometry in complex 3.
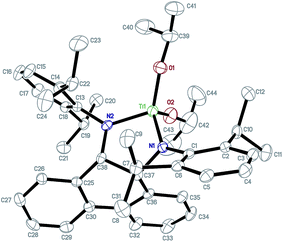 | ||
| Fig. 4 Perspective view of 3 with thermal ellipsoids drawn at 30% probability level. Hydrogens are omitted for clarity. | ||
Lactide polymerization
All titanium complexes were probed as catalysts for the ROP of L-LA or rac-LA. The polymerizations were performed in toluene, and the representative data is recorded in Table 1. The titanium complexes showed low to high activities (71.9–95.6% monomer conversion) in toluene at 70 °C. 1H NMR and GPC were applied to calculate the Mn index of PLA. All polymers had number-average molecular weights (analyzed through GPC28) near the theoretical values (calculated from the monomer/catalyst molar ratio). The molecular weight (Mn) of the polymers propagated almost linearly depending on the increase in the monomer transformation rate, and the polydispersity indices (PDI) of these polymers were relatively low (1.08–1.31, entries 1–8, see e.g. Fig. 5 and Table 1, entry 3). It revealed that the polymerizations in toluene solution were well controlled. It was noted that the activities of these complexes decreased with increasing substituent bulkiness. Complex 1 showed the highest activity (95.6% monomer conversion, Table 1, entry 1) among the three complexes at the same polymerization parameters (Table 1, entries 1–3), which could possibly be attributed to 1 possessing the least steric hindrance among these complexes. Similar observations were also described for Al complexes in previous reports.29| Entry | Cat. | Monomer | T (°C) | t (h) | [LA]0/[Ti]0 | Conv.b (%) | Mn(calcd)c × 10−4 | MnGPCd × 10−4 | PDId | Pr |
|---|---|---|---|---|---|---|---|---|---|---|
| a The polymerizations were performed in toluene solution, [LA]0 = 0.5 mol L−1.b Measured using 1H NMR spectroscopy.c Calculated from the molecular weight of LA × [LA]0/[Ti]0 × conversion + Misopropanolw.d Attained from GPC analysis and calibrated against polystyrene standard. The veritable value of Mn(calcd) could be calculated according to the formula Mn = 0.58MnGPC.e Melt polymerization. | ||||||||||
| 1 | 1 | L-LA | 70 | 12 | 100 | 95.6 | 1.38 | 2.31 | 1.31 | — |
| 2 | 2 | L-LA | 70 | 12 | 100 | 89.4 | 1.29 | 2.09 | 1.25 | — |
| 3 | 3 | L-LA | 70 | 12 | 100 | 73.5 | 1.06 | 1.80 | 1.11 | — |
| 4 | 1 | L-LA | 70 | 5 | 20 | 94.3 | 0.27 | 0.46 | 1.08 | — |
| 5 | 1 | rac-LA | 70 | 12 | 100 | 94.0 | 1.35 | 2.28 | 1.33 | 0.52 |
| 6 | 2 | rac-LA | 70 | 12 | 100 | 90.2 | 1.30 | 2.20 | 1.27 | 0.55 |
| 7 | 3 | rac-LA | 70 | 12 | 100 | 71.9 | 1.04 | 1.71 | 1.17 | 0.59 |
| 8 | 3 | rac-LA | 50 | 17 | 100 | 83.6 | 1.20 | 2.02 | 1.12 | 0.63 |
| 9e | 1 | L-LA | 140 | 3 | 100 | 96.7 | 1.39 | 2.29 | 1.49 | — |
| 10e | 2 | L-LA | 140 | 5 | 100 | 92.4 | 1.33 | 2.21 | 1.40 | — |
| 11e | 3 | L-LA | 140 | 5 | 100 | 82.3 | 1.19 | 2.00 | 1.32 | — |
| 12e | 3 | rac-LA | 140 | 5 | 100 | 81.0 | 1.17 | 1.18 | 1.37 | 0.53 |
| 13e | 1 | L-LA | 140 | 7 | 300 | 91.7 | 3.96 | 6.51 | 1.86 | — |
| 14e | 2 | L-LA | 140 | 7 | 300 | 80.2 | 3.46 | 5.54 | 1.51 | — |
| 15e | 3 | L-LA | 140 | 7 | 300 | 67.3 | 2.91 | 4.92 | 1.35 | — |
 | ||
| Fig. 5 Plots of the PLA Mn and PDI values versus L-LA conversion employing 3, [LA]0/[3]0 = 100, at 70 °C in toluene. | ||
Furthermore, the ligands had a certain ability to influence the PDI of the polymer, and this ability varied with the bulkiness of the ligands. For instance, the PDI decreased from 1.31 to 1.11 with the increasing volume of the substituents “R” from i-propyl to 2,6-di-i-propylphenyl (see Table 1, entries 1 and 3). Complex 3 with a bulky ligand attained the least monomer conversion and lowest PDI, a similar situation was also described in a previous report.30
Kinetics of lactide polymerization
The representative kinetics of the ROP of L-LA (monomer/cat. 3 ratio = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was researched in toluene at 70 °C. The molecular weight (Mn) of the polymer increased linearly with increasing monomer conversion. The PDI value of the polymer remained low (1.07–1.11) (Fig. 5). The plot of ln([LA]0/[LA]t) versus time was linear illustrating that the polymerization rate has a first-order dependency on the monomer when employing 3 for the ROP of L-LA (Fig. 6).
1) was researched in toluene at 70 °C. The molecular weight (Mn) of the polymer increased linearly with increasing monomer conversion. The PDI value of the polymer remained low (1.07–1.11) (Fig. 5). The plot of ln([LA]0/[LA]t) versus time was linear illustrating that the polymerization rate has a first-order dependency on the monomer when employing 3 for the ROP of L-LA (Fig. 6).
 | ||
| Fig. 6 Kinetics of the ROP of L-LA using catalyst 3 at 70 °C in toluene with [LA]0 = 0.5 mol L−1, [cat.]0 = 5 × 10−3 mol L−1, [LA]0/[cat.]0 = 100. | ||
Stereoselective polymerization
Moreover, the formation of poly(rac-LA) (Table 1, entry 8) was also investigated with the homonuclear decoupled 1H NMR spectrum of the methine fragment31 (see Fig. 7). The Pr (ref. 32) value of 0.63, demonstrated that these polymer chains were partially heterotactic. The results showed that the Pr selectivities increased from 0.52 to 0.59 with increasing bulkiness of the substitutents on the ligands at 70 °C (see Table 1, entries 5–7). For complex 3, when reducing the temperature from 70 to 50 °C, the Pr value increased from 0.59 to 0.63 (Table 1, entries 7 and 8).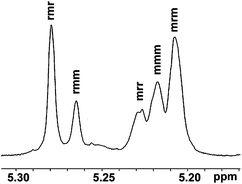 | ||
| Fig. 7 Homonuclear decoupled 1H NMR spectrum of the methine part of poly(rac-LA) using 3 at 50 °C, Pr = 0.63, in CDCl3 (Table 1, entry 8). | ||
Solventless polymerization
In addition, the solventless polymerization (melt polymerization) more and more attracts people’s attention due to reduced pollution. So we tried to polymerize rac-LA via melt polymerization at 140 °C. Under melting conditions, these complexes showed higher activities than those of the polymerization in toluene solution (e.g. Table 1, entries 1, 9 and 13). The highest monomer conversion reached 96.7%.Mechanism of lactide polymerization
For studying the mechanism of the initiation, the end-group analysis of the oligo(lactide), which was synthesized via ROP of LA ([LA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [1] = 20
[1] = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), was analyzed using 1H NMR (Fig. 8). It revealed that the integral ratio of the two peaks at δ = 1.24 ppm, which was attributed to the methyl protons on the isopropoxycarbonyl end, and δ = 4.34 ppm, which was attributed to the methine proton connected to the hydroxyl end, was close to 6/1. This meant the propagating chains were end-capped by an isopropyl ester and a hydroxyl group, and the ROP followed a coordination insertion mechanism.33
1), was analyzed using 1H NMR (Fig. 8). It revealed that the integral ratio of the two peaks at δ = 1.24 ppm, which was attributed to the methyl protons on the isopropoxycarbonyl end, and δ = 4.34 ppm, which was attributed to the methine proton connected to the hydroxyl end, was close to 6/1. This meant the propagating chains were end-capped by an isopropyl ester and a hydroxyl group, and the ROP followed a coordination insertion mechanism.33
 | ||
Fig. 8 1 H NMR spectrum of oligomer of LA ([LA]t![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [1]t = 20 [1]t = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Table 1, entry 4) in CDCl3. 1, Table 1, entry 4) in CDCl3. | ||
Conclusion
In summary, a number of titanium complexes 1–3 were synthesized in good yields. X-ray diffraction analysis revealed that the titanium atom was in a distorted tetrahedral geometry in complex 3. These complexes were employed as catalysts for the polymerization of L-LA and rac-LA. Microstructural analyses of the polymers catalyzed using these complexes revealed that the substituent on the ligand influenced the steric regularity of the polymer and this ability varied with the volume of the ligand. Kinetic studies uncovered that the polymerization of lactide using complex 3 was first-order with respect to the monomer. In addition, these complexes turned out to be efficient catalysts for the ROP of lactides via melt polymerization.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21204082, 51173183, 51021003 and 51321062), Ministry of Science and Technology of China (no. 2011AA02A202), China Postdoctoral Science Foundation (no. 2014M561268), Jilin Science & Technology Department, Science and Technology Development Project (no. 20140204017GX), and Science and Technology Bureau of Changchun City (no. 2013060).Notes and references
- R. Langer and J. P. Vacanti, Science, 1993, 260, 920–926 CAS.
- M. J. Stanford and A. P. Dove, Chem. Soc. Rev., 2010, 39, 486–494 RSC.
- K. Majerska and A. Duda, J. Am. Chem. Soc., 2004, 126, 1026 CrossRef CAS PubMed.
- W. Chen, H. C. Yang, R. Wang, R. Cheng, F. H. Meng, W. X. Wei and Z. Y. Zhong, Macromolecules, 2010, 43, 201–207 CrossRef CAS.
- E. E. Schmitt and R. A. Polistina, U.S. Pat. 3, 463, 158, 1969.
- M. Lahcini, P. M. Castro, M. Kalmi, M. Leskelä and T. Repo, Organometallics, 2004, 23, 4547–4549 CrossRef CAS.
- R. K. Iha, K. L. Wooley, A. M. Nyström, D. J. Burke, M. J. Kade and C. J. Hawker, Chem. Rev., 2009, 109, 5620–5686 CrossRef CAS PubMed.
- A. Alaaeddine, C. M. Thomas, T. Roisnel and J. F. Carpentier, Organometallics, 2009, 28, 1469–1475 CrossRef CAS.
- C.-T. Chen, C.-A. Huang and B.-H. Huang, Dalton Trans., 2003, 3799–3803 RSC.
- V. Poirier, T. Roisnel, J.-F. Carpentier and Y. Sarazin, Dalton Trans., 2009, 9820–9827 RSC.
- C. K. Williams, L. E. Breyfogle, S. K. Choi, W. Nam, V. G. Young Jr, M. A. Hillmyer and W. B. Tolman, J. Am. Chem. Soc., 2003, 125, 11350–11359 CrossRef CAS PubMed.
- X. Pang, X. S. Chen, X. L. Zhuang and X. B. Jing, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 643–649 CrossRef CAS.
- I. J. Blackmore, V. C. Gibson, P. B. Hitchcock, C. W. Rees, D. J. Williams and A. J. P. White, J. Am. Chem. Soc., 2000, 122, 11845–11854 CrossRef.
- L. Wang and H. Ma, Macromolecules, 2010, 43, 6535–6537 CrossRef CAS.
- J.-C. Wu, Y.-Z. Chen, W.-C. Hung and C.-C. Lin, Organometallics, 2008, 27, 4970–4978 CrossRef CAS.
- Y. Takashima, Y. Nakayama, K. Watanabe, T. Itono, N. Ueyama, A. Nakamura, H. Yasuda, A. Harada and J. Okuda, Macromolecules, 2002, 35, 7538–7544 CrossRef CAS.
- S. Gendler, S. Segal, I. Goldberg, Z. Goldschmidt and M. Kol, Inorg. Chem., 2006, 45, 4783–4790 CrossRef CAS PubMed.
- (a) A. F. Douglas, B. O. Patrick and P. Mehrkhodavandi, Angew. Chem., Int. Ed., 2008, 47, 2290–2293 CrossRef CAS PubMed.
- B. Liu, T. Roisnel, L. Maron, J.-F. Carpentier and Y. Sarazin, Chem.–Eur. J, 2013, 19, 3986–3994 CrossRef CAS PubMed.
- B. Liu, D. Cui, J. Ma, X. Chen and X. Jing, Chem.–Eur. J., 2007, 13, 834–845 CrossRef CAS PubMed.
- X. Liu, X. Shang, T. Tang, N. Hu, F. Pei, D. Cui, X. Chen and X. Jing, Organometallics, 2007, 26, 2747–2757 CrossRef CAS.
- (a) N. Spassky, M. Wisniewski, C. Pluta and A. L. Borgne, Macromol. Chem. Phys., 1996, 197, 2627 CrossRef CAS; (b) T. M. Ovitt and G. W. Coates, J. Am. Chem. Soc., 2002, 124, 1316 CrossRef CAS PubMed; (c) C. P. Radano, G. L. Baker and M. R. Smith, J. Am. Chem. Soc., 2000, 122, 1552 CrossRef CAS; (d) Z. Y. Zhong, P. J. Dijkstra and J. Feijen, Angew. Chem., 2002, 114, 4692 (Angew. Chem., Int. Ed., 2002, 41, 4510) CrossRef; (e) H. Du, A. H. Velders, P. J. Dijkstra, J. Sun, Z. Zhong, X. S. Chen and J. Feijen, Chem.–Eur. J., 2009, 15, 9836–9845 CrossRef CAS PubMed; (f) Y. Sarazin, B. Liu, T. Roisnel, L. Maron and J.-F. Carpentier, J. Am. Chem. Soc., 2011, 133, 9069–9087 CrossRef CAS PubMed; (g) I. Yu, A. Acosta-Ramírez and P. Mehrkhodavandi, J. Am. Chem. Soc., 2012, 134, 12758–12773 CrossRef CAS PubMed; (h) A. J. Chmura, M. G. Davidson, C. J. Frankis, M. D. Jones and M. D. Lunn, Chem. Commun., 2008, 1293 RSC.
- (a) B. Gao, R. L. Duan, X. Pang, X. Li, Z. Qu, Z. H. Tang, X. L. Zhuang and X. S. Chen, Organometallics, 2013, 32, 5435–5444 CrossRef CAS; (b) B. Gao, R. Duan, X. Pang, X. Li, Z. Qu, H. Shao, X. Wang and X. Chen, Dalton Trans., 2013, 42, 16334–16342 RSC; (c) Z. H. Tang, X. S. Chen, X. Pang, Y. K. Yang, X. F. Zhang and X. B. Jing, Biomacromolecules, 2004, 5, 965–970 CrossRef CAS PubMed; (d) X. Pang, H. Z. Du, X. S. Chen, X. Wang and X. B. Jing, Chem.–Eur. J., 2008, 14, 3126–3136 CrossRef CAS PubMed; (e) X. Pang, R. L. Duan, X. Li and X. S. Chen, Polym. Chem., 2014, 5, 3894–3900 RSC; (f) X. Pang, R. L. Duan, X. Li, Z. Sun, H. Zhang, X. H. Wang and X. S. Chen, Polym. Chem., 2014, 5, 6857–6864 RSC.
- R. Annunziata, M. Benaglia, M. Caporalem and L. Raimondi, Tetrahedron: Asymmetry, 2002, 13, 2727–2734 CrossRef CAS.
- K. P. Bryliakov, E. A. Kravtsov, D. A. Pennington, S. J. Lancaster, M. Bochmann, H. H. Brintzinger and E. P. Talsi, Organometallics, 2005, 24, 5660–5644 CrossRef CAS.
- A. F. Mason and G. W. Coates, J. Am. Chem. Soc., 2004, 126, 16326–16327 CrossRef CAS PubMed.
- M. G. Thorn, J. E. Hill, S. A. Waratuke, E. S. Johnson, P. E. Fanwick and I. P. Rothwell, J. Am. Chem. Soc., 1997, 119, 8630–8641 CrossRef CAS.
- J. Baran, A. Duda, A. Kowalski, R. Szymanski and S. Penczek, Macromol. Rapid Commun., 1997, 18, 325–333 CrossRef CAS.
- H. Z. Du, A. H. Velders, P. J. Dijkstra, Z. Y. Zhong, X. S. Chen and J. Feijen, Macromolecules, 2009, 42, 1058–1066 CrossRef CAS.
- A. J. Chmura, M. G. Davidson, M. D. Jones, M. D. Lunn, M. F. Mahon, A. F. Johnson, P. Khunkamchoo, S. L. Roberts and S. S. F. Wong, Macromolecules, 2006, 39, 7250 CrossRef CAS.
- K. A. M. Thakur, R. T. Kean, E. S. Hall, J. J. Kolstad, T. A. Lingren, M. A. Doscotch, J. I. Siepmann and E. J. Munson, Macromolecules, 1997, 30, 2422–2428 CrossRef CAS.
- Pr is the probability of racemic linkages between monomer units and is determined from the methine region of the homonuclear decoupled 1H NMR spectrum (Pr + Pm = 1). The expressions for the tetrad concentrations in terms of Pr, assuming Bernoullian statistics and the absence of transesterification, are as follows: [mmm] = (2(1 − Pr)2 + Pr(1 − Pr))/2; [mrm] = (Pr2 + Pr(1 − Pr))/2; [mmr] = [rmm] = (Pr(1 − Pr))/2; [rmr] = Pr2/2. See: B. M. Chamberlain, M. Cheng, D. R. Moore, T. M. Ovitt, E. B. Lobkovsky and G. W. Coates, J. Am. Chem. Soc., 2001, 123, 3229–3238 CrossRef CAS PubMed.
- H. R. Kricheldorf, S. R. Lee and S. Bush, Macromolecules, 1996, 29, 1375–1381 CrossRef CAS.
- G. M. Sheldrick, SHELXTL, Version 5. 1, Siemens Industrial Automation, Inc., 1997 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: X-ray crystallographic data and refinement for complex 3 in CIF format. CCDC 1035591. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra15822a |
| This journal is © The Royal Society of Chemistry 2015 |