Improving the performance of Li–S batteries by reinforced PPy wrapping over acetylene black-coated sulfur†
Wei Qin*a,
Baodong Fangb,
Songtao Luc,
Zhida Wangc,
Yan Chenc,
Xiaohong Wu*c and
Lu Hanc
aSchool of Materials Science and Engineering, Harbin Institute of Technology, Harbin, 150001, PR China. E-mail: qinwei@hit.edu.cn; Fax: +86 451 86402522; Tel: +86 451 86402522
bShanghai Institute of Satellite Engineer, Shanghai, 200240, PR China. E-mail: Fangbd@126.com; Fax: +86 21 34054581; Tel: +86 21 34054581
cDepartment of Chemistry, Harbin Institute of Technology, Harbin, 150001, PR China. E-mail: wuxiaohong@hit.edu.cn; Fax: +86 451 86402522; Tel: +86 451 86402522
First published on 19th January 2015
Abstract
A strategy of reinforced PPy wrapping over an acetylene black-coated sulfur composite (PPy–AB/S) was designed for synthesizing cathode materials of Li–S batteries with improved performance. It was found that the resulting PPy–AB/S cathodes were able to maintain 630 mA h g−1 even after 200 charge–discharge cycles at a rate of 0.5 C.
Due to the ever increasing energy demands and environmental crisis, it is necessary to develop advanced energy storage systems that can retain long-term high-rate discharge for the popularization of electric vehicles (EVs) and hybrid electric vehicles (HEVs).1,2 Among those energy storage systems, the lithium-ion battery has distinct advantages, including high energy density, light weight, wide operating temperature, and no memory effect, and this is applicable for use as the main or sub electrical power supplies of EVs and HEVs.3–5 Though the state-of-the-art lithium-ion battery has improved discharge properties that can maintain its high energy density under large current density, the widespread use is still limited by its low theoretical specific energy densities (e.g. ∼400 W h kg−1 for the LiCoO2/graphite system).6–12 Therefore, the current research interests focus on the exploration of novel lithium battery systems. Lithium–sulfur (Li–S) batteries have received special attentions because of their high theoretical specific capacity (1675 mA h g−1) and high specific energy (2600 W h kg−1) at a moderate voltage of 2.2 V vs. Li/Li+.1,13 In addition, the cathode material elemental sulfur also has other advantages, including its natural abundance, low cost (about %150 per ton), and low environmental impact.14
Despite these promises, the commercial applications of Li–S batteries are still hindered due to the existent several challenges. For example, the inherent low electrical conductivity of sulfur (5 × 10−30 S cm−1), resulting in limited active material utilization efficiency and rate capability. In addition, shuttling of high-order polysulfides between cathode and anode as well as the high solubility of polysulfides intermediates in the electrolyte, leading to limited cycle stability. Additionally, sulfur undergoes severe volumetric expansion/shrinkage during charge and discharge (∼80%), which gradually decreases the mechanical integrity and stability of the electrode over cycles.1,15,16 Therefore, addressing these issues by improving the conductivity of the sulfur cathode and trapping soluble polysulfides within a mechanically stable cathode structure are critical to develop a viable Li–S system.
Over the past decades, various approaches have been made on the fabrication of rationally designed nanostructures and demonstrated a significant improved cyclability and capacity by employment of carbon materials8,17–20 (such as graphene, carbon nanotubes, carbon nanofibers, carbon sphere), polymer additives21–23 (such as PEDOT:PSS, PANI, PVP) or oxides additive24–26 (such as TiO2). It has been reported that the high specific capacities exceeding 1000 mA h g−1 in terms of sulfur have been achieved under low discharge rates (∼0.1 C). Nevertheless, there still existed shuttling effect, especially at high rates, which results in a stable problem under high rates. Most recently, of notable successes, Cui et al. developed Li–S batteries with very high specific capacities of around 700 mA h g−1 at a rate of 1 C and excellent cycle stability.23 In our previous work, we synthesized a cathode made with G–S-CNFs multilayered coaxial nanocomposites, which can deliver an initial capacity of 745 mA h g−1 and maintain ∼273 mA h g−1 even after 1500 charge–discharge cycles at a high rate of 1 C.27 However, the high cost and the elaborate synthetic process involving in varied carbon materials, such as mesoporous carbon, carbon nanotubes, and graphene, are considered to be the drawbacks of the approach.
Acetylene black (AB) is well known as an excellent conductive additive used in plastics, rubber, and batteries, due to its good conductivity, liquid absorbing ability, compressibility and elasticity. To the best of our knowledge, no other conductive additives except AB are used in commercial cells.28 Although the cost may play the main role from the viewpoint of the manufacturers, the inherent advantage of AB nanoparticles over other types of nanoparticles, such as carbon fibers, and large graphites is that they can form extended network structures to provide both conductivity and super mechanical properties at low loadings.29 Considering its application in the Li–S batteries, AB could be incorporated with sulfur by ball-milling or thermal treatment to form sulfur–AB composites as the electrical conductor in the electrode. It was reported that such sulfur–AB composites exhibit poor electrochemical properties.30 Recently, Su Y. S. et al., developed an in situ sulfur deposition route for synthesizing sulfur–carbon composites as the cathode materials for Li–S batteries, involving the precipitation of elemental sulfur at the interspaces between carbon nanoparticles in aqueous solution at room temperature. However, the resulting electrode had low capacity retention, and its electrochemical properties under high charge–discharge rates were not investigated yet.31
Here we report an approach to assemble PPy–AB/sulfur (PPy–AB/S) coaxial structured nanocomposite, in which PPy wraps AB coated sulfur particles. They presented markedly improved cycle stability and capacity and could be used as a cathode for Li–S batteries. In our approach (Fig. 1), nanoscale AB particles were first coated uniformly onto the surface of microscale sulfur particles in an aqueous solution, subsequently, a layer of stacked PPy nanospheres was used to closely wrap around the whole structure in order to enhance the degree of wrapping over sulfur. In this well designed structure, both PPy and AB not only serve as good conducting fillers to improve the overall conductance of the composite, but also trap the polysulfide intermediates. More importantly, PPy has a high absorption ability to restrain the dissolution of polysulfides in the electrolyte. It was found that the electrodes made with such composite were able to deliver a high specific capacity of 1189 mA h g−1 at 0.2 C and still maintain 640 mA h g−1 at 2 C, demonstrating their high-rate capability due to the highly conductive network formed by PPy and AB. Meanwhile, significantly improved stability and capacity at high charge–discharge rate were achieved. PPy–AB/S electrode can deliver a high capacitance of 992 mA h g−1 at the 5th cycle, and maintain 630 mA h g−1 after 200 charge–discharge cycles at 0.5 C, while the Coulombic efficiency remained nearly 100%. These improved rate capability and cycle stability could be attributed to the unique architecture of the composite, that is to say, the synergistic contribution of PPy and AB could enable the electrodes to possess improved electrical conductivity and better ability to trap and absorb the soluble polysulfide intermediates.
 | ||
| Fig. 1 Schematic illustration of the assembled PPy–AB/S composites for improving cathode performance. | ||
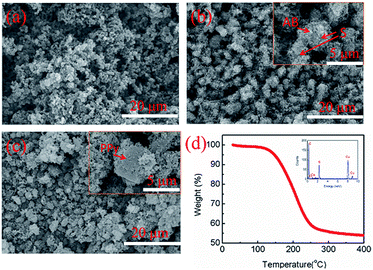
The microstructures of the acetylene black, AB/S composite, and PPy–AB/S composite are shown in Fig. 2. From Fig. 2a, AB has the varying particle sizes between 50 and 100 nm. It also demonstrates spherical morphology without agglomeration, which is beneficial to achieve good electronic conductivity between active materials and/or current collector in the batteries. Fig. 2b presents that the nanoscale AB particles uniformly distributed on the surfaces of the spherical microscale sulfur particles. From the enlarged photo of Fig. 2b (Fig. 2b, inserted), it can be seen that the sulfur particles actually are not fully covered by AB. In order to reinforce the wrapping degree over sulfur, PPy stacked by PPy nanospheres was used to fill the gaps among AB. As can be seen from Fig. 2c and its enlarged photo (Fig. 2c, inserted), the diameter of the spherical composite was remarkably increased, indicating that a uniform PPy layer was formed over the spherical composite, which will effectively enhance the conductivity of the composite material. As a result, the obtained uniform structure provides not only excellent electron pathways for the insulating sulfur but also many adsorbent points for the soluble polysulfides to avoid their loss into the electrolyte. The energy-dispersive spectroscopy (EDS) of the PPy–AB/S composites (Fig. 2d, inserted) clearly shows the existence of sulfur. The actual content of sulfur in the composites were determined by thermo gravimetric analysis (TGA) conducted under nitrogen environment (Fig. 2d) to be 45%, which is similar to that as determined from the precursors.
A series of electrochemical tests were carried out to study the effects of the PPy–AB/S composites on the cyclic performance of the electrode. The cathodes for Li–S battery testing were prepared by slurry coating of PPy–AB/S and PVDF binder on Al foil. All the test batteries were assembled as coin cells (type 2032) with a small piece of lithium foil used as the counter electrodes. Fig. 3 compares the cyclabilities of the AB/S composite electrodes with and without PPy coating at 0.5 C (where 1 C corresponds to a current density of 1675 mA g−1) within 1.5–3.0 V (vs. Li/Li+). Both of these two electrodes have similar sulfur content of ∼40.5 wt%. Obviously, the PPy–AB/S composite electrode exhibited more stable cycling performance and higher discharge capacity than the AB/S composite electrode over 200 cycles. Based on the discharge results, the PPy–AB/S composite cathode was able to deliver an initial discharge capacity of 847 mA h g−1 (calculation based on the weight of S), and which gradually increased until the 5th cycle (992 mA h g−1). This behavior could be associated with the activation of the PPy–AB/S electrode, that is, it takes some time for the electrolyte to flood the internal surfaces of the PPy–AB/S electrode. Thereafter, the deeply buried sulfur and disulfide bonds can contact with the electrolyte and become electrochemically active. Subsequently, the capacities almost stabilized and demonstrated little fading upon extended cycling. In summary, the electrode can exhibit a reversible and comparable capacity of 630 mA h g−1 after 200 cycles, corresponding to a capacity retention of 63.5% (of its highest capacity of 992 mA h g−1). At the same time, the electrode exhibited well-overlapped and flat plateaus as shown in Fig. 4, suggesting its good stability and reversibility. Furthermore, the electrode displayed a high Coulombic efficiency at around 100%. On the basis of such superior cyclic stability, it is reasonable to conclude that the synergistic effects of PPy and AB can effectively improve the cycle stability of sulfur probably through confining and absorbing the polysulfide intermediates, and provide better conductivity and mechanical support to accommodate the volume changes during charge and discharge. For the control sample without PPy wrapping, the AB/S electrode had a higher initial discharge capacity of about 1094 mA h g−1 than that of the PPy–AB/S, indicating that the electrolyte can flood the surface of sulfur quickly through those unwrapped pores. With continued cycling, the AB/S electrode was able to stabilize at 366 mA h g−1 after 200 cycles, corresponding to a capacity retention of 33.5%. All the results show that the cycling performance and discharge capacity can be significantly enhanced by the reinforced PPy wrapping on AB/S.
 | ||
| Fig. 3 Comparison of specific capacity and Coulombic efficiency as a function of cycle numbers for electrodes assembled with and without PPy wrapping. | ||
 | ||
| Fig. 4 Charge–discharge profiles at different cycle numbers as labeled for the PPy–AB/S cathode for Li–S battery at a current density of 0.5 C. | ||
We further compared the discharge–charge voltage profiles of the PPy–AB/S and AB/S electrodes at various current densities from 0.2 C to 2 C between 3.0 and 1.5 V. All of the discharge–charge curves demonstrated similar plateaus, as shown in Fig. 5. In detail, two plateaus at 2.35 and 2.05 V were clearly observed during the discharge process, which correspond to the formation of long-chain lithium polysulfides (Li2Sx, 4 ≤ x ≤ 8) and short-chain lithium polysulfides (such as Li2S2 and Li2S), respectively.
 | ||
| Fig. 5 Charge–discharge profiles at various current densities as labeled for the PPy–AB/S cathode for Li–S battery. | ||
The rate capability of the PPy–AB/S composite electrode at various current densities from 0.2 C to 2 C was also investigated and was presented in Fig. 6. As the current increases, the capacity of the PPy–AB/S composites electrode decreased slowly from the reversible 1130 mA h g−1 at 0.2 C to 928, 688 and 633 mA h g−1 at 0.5 C, 1 C and 2 C, respectively. Compared with the PPy–AB/S composite electrode, the AB/S composite electrode exhibited a lower capacity. Importantly, a reversible capacity of 642 mA h g−1 was obtained when the current was switched back to 1 C, which is close to the initial capacity (688 mA h g−1), indicating a highly reversible and efficient electrode enabled by the reinforced PPy wrapping.
 | ||
| Fig. 6 Comparison of the capacity vs. current density (0.2 C, 0.5 C, 1 C and 2 C) of the cathodes assembled with (PPy–AB/S) and without (AB/S) the reinforced PPy wrapping. | ||
Conclusions
In summary, we developed a method of reinforced PPy wrapping over the AB/S composites for high-performance Li–S batteries. In this well-designed structure, sulfur was not only coated by AB but also reinforced by PPy. Owning to the unique core–shell architecture, PPy–AB/S was able to deliver high specific capacity, high retention ratio at increased charging–discharging rates with outstanding stability. This work proposed a general strategy for improving performance of sulfur cathodes in terms of having high capacity and cycle stability during cycling from the viewpoint of the reinforced wrapping. In addition, we expect that this strategy will cover general interests and influence many other fields.Acknowledgements
Financial support from the Nature Science Foundation of China (no. 51078101, 51173033), the Fundamental Research Funds for the Central Universities (no. HIT.BRETIII.201224 and 201312) and Shanghai key Laboratory of Deep Space Exploration Technology (no. 13dz2260100).Notes and references
- P. G. Bruce, S. A. Freunberger, L. J. Hardwick and J. M. Tarascon, Nat. Mater., 2012, 11, 19–29 CrossRef CAS PubMed.
- Y. S. Su, Y. Z. Fu, B. K. Guo, S. Dai and A. Manthiram, Chem.–Eur. J., 2013, 19, 8621–8626 CrossRef CAS PubMed.
- A. Manthiram, J. Phys. Chem. Lett., 2011, 2, 176–184 CrossRef CAS.
- B. C. Duan, W. K. Wang, A. B. Wang, Z. B. Yu, H. L. Zhao and Y. S. Yang, J. Mater. Chem. A, 2014, 2, 308–314 CAS.
- X. F. Li and C. L. Wang, J. Mater. Chem. A, 2013, 1, 165–182 CAS.
- Z. Li, L. X. Yuan, Z. Q. Yi, Y. Liu, Y. Xin, Z. L. Zhang and Y. H. Huang, Nanoscale, 2014, 6, 1653–1660 RSC.
- D. Li, F. Han, S. Wang, F. Cheng, Q. Sun and W. C. Li, ACS Appl. Mater. Interfaces, 2013, 5, 2208–2213 CAS.
- G. Y. Zheng, Y. Yang, J. J. Cha, S. S. Hong and Y. Cui, Nano Lett., 2011, 11, 4462–4467 CrossRef CAS PubMed.
- Y. S. Jung, P. Lu, A. S. Cavanagh, C. Ban, G. H. Kim, S. H. Lee, S. M. George, S. J. Harris and A. C. Dillon, Adv. Energy Mater., 2013, 3, 213–219 CrossRef CAS.
- C. D. Liang, N. J. Dudney and J. Y. Howe, Chem. Mater., 2009, 21, 4724–4730 CrossRef CAS.
- M. K. Song, Y. G. Zhang and E. J. Cairns, Nano Lett., 2013, 13, 5891–5899 CrossRef CAS PubMed.
- M. K. Song, E. J. Cairns and Y. G. Zhang, Nanoscale, 2013, 5, 2186–2204 RSC.
- F. G. Sun, J. T. Wang, D. H. Long, W. M. Qiao, L. C. Ling, C. X. Lv and R. Cai, J. Mater. Chem. A, 2013, 1, 13283–13289 CAS.
- X. Y. Tao, X. R. Chen, Y. Xia, H. Huang, Y. P. Gan, R. Wu, F. Chen and W. K. Zhang, J. Mater. Chem. A, 2013, 1, 3295–3301 CAS.
- H. Xu, Y. F. Deng, Z. C. Shi, Y. X. Qian, Y. Z. Meng and G. H. Chen, J. Mater. Chem. A, 2013, 1, 15142–15149 CAS.
- C. Barchasz, F. Mesguich, J. Dijon, J. C. Lepretre, S. Patoux and F. Alloin, J. Power Sources, 2012, 211, 19–26 CrossRef CAS PubMed.
- F. F. Zhang, X. B. Zhang, Y. H. Dong and L. M. Wang, J. Mater. Chem., 2012, 22, 11452–11454 RSC.
- Y. S. Su and A. Manthiram, Chem. Commun., 2012, 48, 8817–8819 RSC.
- M. S. Park, J. S. Yu, K. J. Kim, G. Jeong, J. H. Kim, T. Yim, Y. N. Jo, U. Hwang, S. Kang, T. Woo, H. Kim and Y. J. Kim, RSC Adv., 2013, 3, 11774–11781 RSC.
- L. W. Ji, M. M. Rao, H. M. Zheng, L. Zhang, Y. C. Li, W. H. Duan, J. H. Guo, E. J. Cairns and Y. G. Zhang, J. Am. Chem. Soc., 2011, 133, 18522–18525 CrossRef CAS PubMed.
- L. Wang, X. M. He, J. J. Li, J. Gao, J. W. Guo, C. Y. Jiang and C. R. Wan, J. Mater. Chem., 2012, 22, 22077–22081 RSC.
- Y. Yang, G. H. Yu, J. J. Cha, H. Wu, M. Vosgueritchian, Y. Yao, Z. A. Bao and Y. Cui, ACS Nano, 2011, 5, 9187–9193 CrossRef CAS PubMed.
- W. Y. Li, G. Y. Zheng, Y. Yang, Z. W. Seh, N. Liu and Y. Cui, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 7148–7153 CrossRef CAS PubMed.
- Z. W. Seh, W. Y. Li, J. J. Cha, G. Y. Zheng, Y. Yang, M. T. McDowell, P. C. Hsu and Y. Cui, Nat. Commun., 2013, 4, 1331, DOI:10.1038/ncomms2327.
- C. S. Kim, A. Guerfi, P. Hovington, J. Trottier, C. Gagnon, F. Barray, A. Vijh, M. Armand and K. Zaghib, Electrochem. Commun., 2013, 32, 35–38 CrossRef CAS PubMed.
- J. Y. Li, B. Ding, G. Y. Xu, L. R. Hou, X. G. Zhang and C. Z. Yuan, Nanoscale, 2013, 5, 5743–5746 RSC.
- S. T. Lu, Y. W. Cheng, X. H. Wu and J. Liu, Nano Lett., 2013, 13, 2485–2489 CrossRef CAS PubMed.
- G. Liu, H. Zheng, S. Kim, Y. Deng, A. M. Minor, X. Song and V. S. Battaglia, J. Electrochem. Soc., 2008, 155, A887–A892 CrossRef CAS PubMed.
- W. P. Tang, J. Mater. Chem., 2004, 14, 3457–3461 RSC.
- B. Zhang, C. Lai, Z. Zhou and X. P. Gao, Electrochim. Acta, 2009, 54, 3708–3713 CrossRef CAS PubMed.
- Y. S. Su and A. Manthiram, Electrochim. Acta, 2012, 77, 272–278 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra15796f |
| This journal is © The Royal Society of Chemistry 2015 |