Multifunctional reversibly sealable microfluidic devices for patterned material deposition approaches†
Abstract
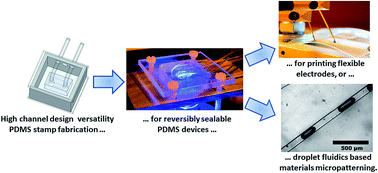
We present a concept to produce reversibly sealable polydimethylsiloxane (PDMS) based microfluidic devices with versatile channel designs, withstanding pressures up to 600 kPa. A novel fabrication strategy, namely the casting of a secondary PDMS casing around the initial channel system allows diverting the tubing attached to the channels sideways so that a simple mounting assembly can be used to press the fluidic chip onto virtually any type of substrate. We demonstrate the functionalities of the developed setup at a proof-of-concept level by direct printing of electronic interconnects onto flexible substrates in a single step. As a second application, we generate uniquely shaped polymer structures when combining the presented technique with droplet microfluidics using a UV-curable adhesive and water as continuous and dispersed phases, respectively. We believe the developed approach has a plethora of applications and a clear perspective of being used for cost-efficient and multifunctional designs of novel classes of materials and devices in diverse areas from electronics to biotechnology.

 Please wait while we load your content...
Please wait while we load your content...