An experimental and theoretical study of the kinetics of the reaction between 3-hydroxy-3-methyl-2-butanone and OH radicals†
Angappan Mano Priyaa,
Gisèle El Dib*b,
Lakshmipathi Senthilkumara,
Chantal Sleimanb,
Alexandre Tomasc,
André Canosab and
Abdelkhaleq Chakird
aDepartment of Physics, Bharathiar University, Coimbatore-641046, Tamil Nadu, India
bInstitut de Physique de Rennes, (IPR), UMR 6251 du CNRS - Université de Rennes 1, Bat. 11C, Campus de Beaulieu, 35042 Rennes Cedex, France. E-mail: gisele.eldib@univ-rennes1.fr; Fax: +33 2 23 23 67 86; Tel: +33 2 23 23 56 80
cMines Douai, Département Sciences de l'Atmosphère et Génie de l'Environnement, F-59508 Douai, France
dUniversité de Reims, Laboratoire GSMA-UMR 6089 CNRS, Campus Moulin de la Housse, BP 1039, 51687 Reims Cedex 02, France
First published on 6th March 2015
Abstract
Absolute experimental and theoretical rate constants are determined for the first time for the reaction of 3-hydroxy-3-methyl-2-butanone (3H3M2B) with OH radicals as a function of temperature. Experimental studies were carried out over the temperature range of 277 to 353 K and the pressure range of 5 to 80 Torr, by using a cryogenically cooled cell coupled to the PLP-LIF technique. OH radicals were generated for the first time from the photodissociation of the reactant 3H3M2B at 266 nm and the OH formation yield in 3H3M2B photolysis at 266 nm was measured under our experimental conditions. In addition, the reaction of 3H3M2B with OH radicals was studied theoretically by using the Density Functional Theory (DFT) method under three hydrogen abstraction pathways. According to these calculations, H-atom abstraction occurs more favourably from the methyl group adjacent to the hydroxyl group with a small barrier height. The calculated theoretical rate constants are in good agreement with the experimental data over the temperature range of 278 to 1000 K. No significant temperature dependence can be observed although a very slight effect was observed within the error bars.
1. Introduction
Ketones are ubiquitous atmospheric key components that are produced in the oxidation of Volatile Organic Compounds (VOCs) emitted from both anthropogenic and biogenic sources.1,2 Ketones are also a major class of organic chemicals and solvents emitted directly into the Earth's atmosphere. They have various uses in chemistry, medicine and industry, as solvents for resins, cellulose, lacquers and for making perfumes, dyes, flavourings, chloroform and plastics.3,4 In addition to the above applications, ketones are also used as fuel tracers for monitoring fuel properties, such as concentration, temperature, density, pressure, velocity and act as fuel additives to reduce soot emissions.5–8 Moreover, these species are formed extensively in the atmosphere with other organic compounds and represent important intermediates in the oxidation and combustion of hydrocarbons and biofuels.4 In the paper we will focus on 3-hydroxy-3-methyl-2-butanone (3H3M2B) which belongs to the family of hydroxyketones. This species is used to produce optical plastics, photodegradable plastics and fire-resistant rubbers.3As for other VOCs present in the atmosphere, hydroxyketones in the gas phase may undergo two different processes: photolysis under solar radiation and chemical reactions with atmospheric photo oxidants9–12 (OH, Cl, NO3, O3). Among these radicals, the hydroxyl radical (OH) is a highly reactive free radical that belongs to the general class of species known as Reactive Oxygen Species (ROS) and it has an atmospheric lifetime of few seconds. It is one of the most important species influencing chemical processes in the atmosphere. Therefore, kinetic investigations of the OH radical reaction with VOCs are essential for the evaluation of their significance in air pollution.
In recent years, the investigation of gas phase oxidation of hydroxyketones has received much attention in both experimental and theoretical studies, because only a few kinetic models have been studied so far. Based on the literature review, there are three studies that report experimental rate constant values only at atmospheric pressure for chemical reactions of 3H3M2B with atmospheric photooxidants9,12,13 by using a relative rate technique. The objective of the present study is to provide thermodynamical properties and kinetic parameters of the reaction of 3H3M2B with OH radicals. This work reports the first absolute rate constants for the reactions of 3H3M2B with OH in the gas phase, as a function of pressure and temperature and the first computational kinetic data on this reaction. Moreover, here, 3H3M2B was used as the OH precursor and the OH formation yield from the photodissociation of 3H3M2B at 266 nm has been measured for the first time. The obtained results enable a better understanding of the importance of the studied reaction as a removal process for 3H3M2B under atmospheric conditions in the gas phase and to evaluate its role in the chemistry of the atmosphere.
2. Experimental
The kinetics of the reaction of OH with 3H3M2B was studied at IPR-Rennes (France) in the gas phase by using a cryogenically cooled cell coupled to the Pulsed Laser Photolysis-Laser Induced Fluorescence (PLP-LIF) technique. The experiments were carried out in the temperature range of 277 to 353 K and over the pressure range of 5 to 80 Torr.A part of the experimental set up has been described in details in a previous study,11 therefore only a brief description is given here. The reactor is a stainless steel cryogenically cooled cell (320 cm3), 65 cm in length and 2.5 cm in diameter. The temperature regulation is provided by water, circulating in a copper tube surrounding the cell and commanded by a thermocryostat. Helium was used as the main carrier gas; it was introduced inside the reactor 25 cm upstream of the detection zone. Two thermocouples positioned at the entrance of the gas and upstream of the detection zone were used to measure the temperature inside the cell. Vapours of 3H3M2B were premixed with helium in a 20 L bulb to form a (0.5–1) % mixture at a total pressure of about 750 Torr. The concentration of 3H3M2B introduced into the cell was calculated from the dilution rate in the bulb, the mass flow rates, the temperature and the total pressure in the cell. The mass flow rates were measured by using calibrated mass flow controllers MKS (100–5000 sccm) and the pressure was measured by using (10–1000 Torr) MKS capacitance manometers.
The rate constants were determined under pseudo-first order conditions where the concentration of 3H3M2B was in large excess compared to that of OH. In this work, OH radicals were generated, to our knowledge for the first time, from the photodissociation of 3H3M2B at 266 nm and the OH formation yield was measured, according to the procedure described later in this paragraph. A Nd:YAG laser emitting at 266 nm was used for the production of OH radicals in the ground state X2Π, ν′′ = 0. This photolysis beam passed longitudinally through the cell to photodissociate 3H3M2B and thus to generate OH radicals. The laser fluence was typically of 30 ± 1 mJ per pulse per cm2. The determination of the rate constant of the studied reaction required the monitoring of the temporal OH decay due to its reaction with 3H3M2B. For this purpose, a probe laser beam was generated by using a frequency-doubled dye laser (Continuum ND 6000) pumped by a Nd:YAG laser (Spectra Physics, GCR 230) emitting at 532 nm. This probe laser crosses perpendicularly with the photolysis beam through the cell to excite the OH radicals at 281.99 nm (electronic transition A2Σ+ – X2Π (1, 0)). The OH fluorescence (transition A2Σ+ – X2Π (1, 1) and (0, 0) at ca. 310 nm) was then detected by a photomultiplier tube (PMT) perpendicular to both the laser beams and gas flows and set in the middle of the cell. The output pulse of the PMT was then integrated for a preset period by a gated charge integrator. In order to avoid the accumulation of photolysis or reaction products, the experiments were carried out under flow conditions so that the gas mixture in the cell was renewed between two consecutive photolysis pulses.
Typically 4 to 6 fluorescence decays were averaged to generate OH concentration vs. time profiles. Both the photolysis and the fluorescence lasers have repetition rates of 10 Hz. In Fig. 1, a typical fluorescence OH decay signal is shown for a given 3H3M2B concentration, as a function of the delay time between the photolysis and probe lasers according to the following relation:
| [OH] = [OH]0e−kfirstt | (1) |
| kfirst = k[3H3M2B] | (2) |
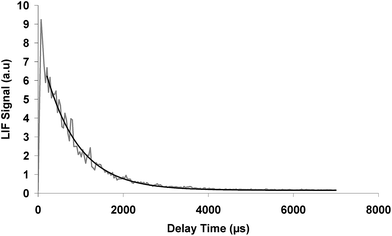 | ||
| Fig. 1 OH radical kinetic decay as a function of the delay time between the pulses from the photolysis and probe lasers. P = 40 Torr, T = 295 K, [3H3M2B] = 3.2 × 1014 molecule per cm3. | ||
| T/K | P/Torr | Total flow/cm3 min−1 atm1 | Linear velocity/cm s−1 | Number of runs | kexpa/10−13 cm3 molecule−1 s−1 (±2σ) | kpredictedc/10−13 cm3 molecule−1 s−1 |
|---|---|---|---|---|---|---|
a Average experimental values where 2σ is two standard deviations.b Average experimental value at 295 K at different pressures.c Predicted values using the temperature expression: k(T)theory = (0.17 ± 0.03) × 10−13 (T/300)1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp((1226 ± 55)/T). exp((1226 ± 55)/T). |
||||||
| 277.5 | 10 | 1100 | 285 | 6 | 5.5 ± 0.8 | 12.4 |
| 288 | 10 | 2000 | 517 | 3 | 4.7 ± 0.7 | 11.2 |
| 295 | 5 | 500 | 260 | 2 | 5.2 ± 0.8 | |
| 295 | 10 | 1000 | 258 | 4 | 4.0 ± 0.8 | |
| 295 | 20 | 2000 | 258 | 2 | 4.7 ± 1.1 | |
| 295 | 40 | 4500 | 290 | 5 | 5.2 ± 0.8 | |
| 295 | 80 | 7500 | 227 | 2 | 4.9 ± 0.5 | |
| 4.8 ± 0.8b | 10.5 | |||||
| 313 | 10 | 1400 | 362 | 2 | 4.5 ± 0.8 | 9.1 |
| 333 | 10 | 1400 | 362 | 4 | 4.4 ± 1.1 | 7.9 |
| 353 | 10 | 2000 | 517 | 2 | 4.3 ± 1.0 | 7.1 |
The OH formation yield in the photolysis of 3H3M2B at 266 nm was determined relative to that of OH in the photolysis of H2O2 using the LIF technique described above over the pressure range of 5 to 80 Torr. Vapours of H2O2 were introduced into the cell by using a bubbler system similar to that described in a previous study.11 The concentration of H2O2 in the cell was calculated from the vapour pressure of H2O2, the mass flow rates, the temperature and the pressure in the reactor and the bubbler. OH signals from 3H3M2B and H2O2 photolysis were measured in back-to-back experiments while keeping the photolysis laser fluence constant, as follows: a known concentration of H2O2 was introduced into the cell and the OH temporal decay due to its reaction with H2O2 was recorded. The initial LIF signal was determined by extrapolation at the initial time of the fitted exponential decay. H2O2 was then replaced by 3H3M2B and the OH LIF signal was determined by extrapolation at the initial time as in the case of H2O2. The OH formation yield in the photolysis of 3H3M2B at 266 nm was therefore obtained using the following relationship:
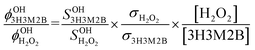 | (3) |
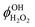 (=2) are the OH formation yields in the photolysis of 3H3M2B and H2O2 at 266 nm, respectively. SOH3H3M2B and
(=2) are the OH formation yields in the photolysis of 3H3M2B and H2O2 at 266 nm, respectively. SOH3H3M2B and 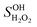 are the OH signals at the initial time from 3H3M2B and H2O2 photolysis, respectively. σ3H3M2B (5.33 × 10−20 cm2 per molecule)14 and σH2O2 (4.2 × 10−20 cm2 per molecule)15 are the absorption cross sections of 3H3M2B and H2O2 at 266 nm, respectively and [3H3M2B] and [H2O2] are the concentrations of 3H3M2B and H2O2 introduced into the cell, respectively. The background signal and scattered light were subtracted from the LIF signals.
are the OH signals at the initial time from 3H3M2B and H2O2 photolysis, respectively. σ3H3M2B (5.33 × 10−20 cm2 per molecule)14 and σH2O2 (4.2 × 10−20 cm2 per molecule)15 are the absorption cross sections of 3H3M2B and H2O2 at 266 nm, respectively and [3H3M2B] and [H2O2] are the concentrations of 3H3M2B and H2O2 introduced into the cell, respectively. The background signal and scattered light were subtracted from the LIF signals.
Helium > 99.995%-Air Liquide was used without further purification. 3H3M2B (purity > 95%) was from Sigma Aldrich. It was further purified before use. The H2O2 solution (50% in water) obtained from Sigma Aldrich was concentrated by bubbling helium through the solution to remove water for several days prior to use and constantly during the course of the experiments.
3. Computational details
The computational work was carried out in the Department of Physics at Bharathiar University, India. The molecular structures, harmonic vibrational frequencies of all stationary points involved in the pathways for the reaction of 3H3M2B with OH were optimized using the Density Functional Theory method (DFT) described in details by Kohn et al.16 and widely used in theoretical calculations such as thermodynamic and kinetic studies.17,18 The geometries were optimized and vibrational frequencies were calculated using density functional method BH&HLYP19,20 with 6-311++G(d,p) basis set. To verify the nature of stationary point, harmonic vibrational frequencies were calculated for all the structures. The transition state of the complexes has only one imaginary frequency, thus confirming their location as maxima in one reaction coordinate. The Minimum Energy Path (MEP) was constructed with intrinsic reaction co-ordinate (IRC)21,22 calculation in order to verify whether the transition state connects exactly with the designated local minima. Earlier gas phase kinetic studies have shown that BH&HLYP functionals are the best compromise for calculating accurate barrier heights among all density functional theory based methods. They are as well accurate for predicting reliable low-level potential energy surface information (geometries, frequencies, and reaction path) of the stationary points.18,23–26 In addition to calculation using the BH&HLYP functionals, ΔE, ΔH and ΔG for the three pathways were calculated by MP2 (ref. 27 and 28) level of theory with the same basis set, as well.There are several methods that can be used to calculate rate constants.29 In this work, the kinetics of all the reaction pathways were evaluated using the canonical variational transition state theory coupled with small curvature tunneling (CVT/SCT) over the temperature range of 278 to 1000 K using BH&HLYP functional. Through canonical variational transition state theory (CVT)30–32 the rate constant at temperature T is given by the following relation:
| kCVT(T) = minskGT(T, s) | (4) |
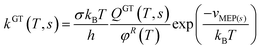 | (5) |
The equilibrium constant (Kc in concentration units) for the reactions in equilibrium is evaluated using the standard formulae:33
| Kc = Kp(R′T)−Δn | (6) |
R′T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kp = −ΔGT0 Kp = −ΔGT0
| (7) |
4. Results and discussion
4.1 Experimental kinetic study and OH formation yield
Absolute rate constants of the reaction of 3H3M2B with OH radicals were determined in this study over the temperature range of 277 to 353 K and the pressure range of 5 to 80 Torr. Several runs were carried out at given temperature and pressure. For each run, an individual rate constant was obtained from 4 to 6 averaged fluorescence decays according to eqn (2) from the plots of kfirst vs. [3H3M2B] such as the example displayed in Fig. 2. The uncertainties on these individual values are about 20% which include both systematic and statistical errors. The systematic errors originate from the difficulties in handling and measuring the concentrations of the studied compound with good accuracy due to its low vapour pressure and impurities. In order to minimize uncertainties coming from theses sources, 3H3M2B was purified by repeated freeze–pump–thaw cycles before use and the pressure of 3H3M2B introduced in the mixing bulb and in the cell was widely lower than its vapour pressure at the working temperature (13.5 Torr) which was measured in this work. The statistical errors originate from the least-squares analysis of the plots of kfirst vs. [3H3M2B] as displayed in Fig. 2, multiplied by the Student's t-factor appropriate for the 95% confidence interval and the number of degrees of freedom. Average rate constants of these individual values are calculated under given conditions (temperature and pressure) and listed in Table 1 where the reported uncertainties are two standard deviations. A variation from 5 to 80 Torr of total pressure had no effect on the rate constants obtained at room temperature within the experimental errors.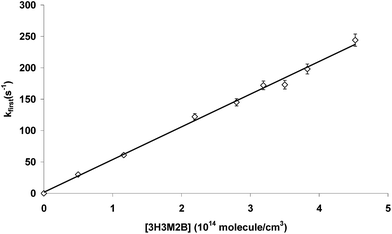 | ||
| Fig. 2 Plot of the pseudo-first-order decay rates as a function of 3H3M2B concentration: P = 40 Torr, T = 295 K, [3H3M2B] = (0.49–4.52) × 1014 molecule per cm3. | ||
The OH formation yield in the 3H3M2B photolysis at 266 nm was measured relative to that generated in the photolysis of H2O2 as explained before. The least squares fit of  vs.
vs.  obtained at different total pressures, yield slopes very close to each other: (0.19 ± 0.03) at 5 Torr, (0.19 ± 0.01) and (0.21 ± 0.05) both at 10 Torr and (0.20 ± 0.03) at 80 Torr. In Fig. 3, the data for different pressures are gathered in the same graph and at the same scale. The obtained slope is (0.20 ± 0.01) yielding a formation yield ϕOH3H3M2B of (32 ± 6) % under our experimental conditions. For each point in Fig. 3, the error bar indicates the statistical errors due to the fit of the corresponding experimental OH decay profile by the exponential function according to eqn (1). The overall error on the quantum yield includes statistical errors (5%) on the slope of Fig. 3 due to the least squares fit and systematic errors (15%). These errors originate mainly from the measurements of H2O2 and 3H3M2B concentrations and the absorption cross sections of 3H3M2B at 266 nm. No data are available in the literature concerning the uncertainty on the absorption cross section of H2O2 at 266 nm and the quantum yield for the production of OH from H2O2 at this wavelength.
obtained at different total pressures, yield slopes very close to each other: (0.19 ± 0.03) at 5 Torr, (0.19 ± 0.01) and (0.21 ± 0.05) both at 10 Torr and (0.20 ± 0.03) at 80 Torr. In Fig. 3, the data for different pressures are gathered in the same graph and at the same scale. The obtained slope is (0.20 ± 0.01) yielding a formation yield ϕOH3H3M2B of (32 ± 6) % under our experimental conditions. For each point in Fig. 3, the error bar indicates the statistical errors due to the fit of the corresponding experimental OH decay profile by the exponential function according to eqn (1). The overall error on the quantum yield includes statistical errors (5%) on the slope of Fig. 3 due to the least squares fit and systematic errors (15%). These errors originate mainly from the measurements of H2O2 and 3H3M2B concentrations and the absorption cross sections of 3H3M2B at 266 nm. No data are available in the literature concerning the uncertainty on the absorption cross section of H2O2 at 266 nm and the quantum yield for the production of OH from H2O2 at this wavelength.
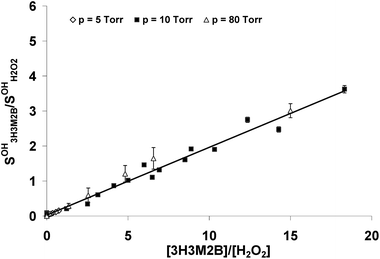 | ||
| Fig. 3 Plot of the ratio of the initial OH signals from the photolysis of 3H3M2B and H2O2 at 266 nm vs. the ratio of the concentrations at three different pressures. | ||
4.2 Computational results
The reaction of OH with 3H3M2B is expected to proceed through the abstraction of hydrogen in three ways, namely, from the hydroxyl group (OH), and from one of the methyl groups adjacent to both the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) group or to the hydroxyl (OH) group. The three hydrogen abstraction channels shown below are named as, pathway 1 (H-atom abstraction from the hydroxyl group of 3H3M2B), pathway 2 (abstraction from the methyl group adjacent to the carbonyl group) and pathway 3 (abstraction from the methyl group adjacent to the hydroxyl group).
O) group or to the hydroxyl (OH) group. The three hydrogen abstraction channels shown below are named as, pathway 1 (H-atom abstraction from the hydroxyl group of 3H3M2B), pathway 2 (abstraction from the methyl group adjacent to the carbonyl group) and pathway 3 (abstraction from the methyl group adjacent to the hydroxyl group).
The optimized molecular structures of the reactant (R), the reactant complex (RC), transition states (TS), product complexes (PC) and products (P) of 3H3M2B along with OH radicals of all the three pathways calculated at BH&HLYP/6-311+G(d,p) level of theory are shown in Fig. S1.† The barrier height, enthalpy and Gibbs free energies of the three hydrogen abstraction channels are calculated at BH&HLYP and MP2 level of theory and summarized in Table 2. The obtained results show that the BH&HLYP thermodynamic values are comparable with those obtained by using the MP2 level. For the barrier height, the difference in value between the two methods is 0.74 kcal mol−1 and 0.51 kcal mol−1 for TS2 and TS3 respectively. The calculations did not converge to determine the thermodynamic parameters of transition state TS1, therefore only the BH&HLYP method was used here to calculate the rate constants.
| Stationary points | BH&HLYP/MP2/6-311+G(d,p) | ||
|---|---|---|---|
| ΔE | ΔH | ΔG | |
| a ( )Values in parenthesis were calculated using MP2/6-311+G(d,p), * indicates structure not converged in MP2 level of theory. | |||
| 3H3M2B + OH | 0 | 0 | 0 |
| RC | −7.49(−5.93) | −6.84(−7.81) | −6.25(−10.21) |
| TS1 | 4.13(*) | 2.02(*) | 6.71(*) |
| PC1 | −17.48(−14.19) | −16.43(−13.14) | −15.26(−12.14) |
| P1 | −16.43(−13.79) | −15.40(−12.80) | −13.55(−14.19) |
| TS2 | 2.42(3.16) | 0.90(1.28) | 4.52(4.71) |
| PC2 | −30.50(−30.45) | −29.15(−28.77) | −26.97(−26.95) |
| P2 | −27.39(−27.60) | −26.76(−26.63) | −26.15(−27.31) |
| TS3 | 1.59(2.10) | 0.37(0.22) | 3.74(3.77) |
| PC3 | −19.76(−24.72) | −19.96(−25.01) | −19.83(−25.38) |
| P3 | −19.48(−24.47) | −19.79(−24.87) | −19.96(−25.17) |
In this study one reactant complex (RC) and three product complexes (PC) are identified. (RC) remains the same for all the three pathways, characterised with an intermolecular hydrogen bond formed between the hydrogen atom in the methyl group of 3H3M2B adjacent to the OH group and the OH radical. Further, we observe an intramolecular hydrogen bond formed between the oxygen atom of the carbonyl group and the hydrogen atom of the hydroxyl group within 3H3M2B.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group. This bond has a length of 1.982 Å, while the hydrogen bond formed between the OH radical and the methyl group has a bond distance of 2.658 Å. The reactant complex (RC) proceeds toward the product (PC1) through the transition state (TS1) with an energy barrier of 4.13 kcal mol−1. The product complex (PC1) is formed between the oxygen atom of the carbonyl group and the water molecule with a bond distance of 1.925 Å. Subsequently, the product complex breaks into product (P1) and a water molecule due to the high electron affinity of the oxygen atom (O3) in the hydroxyl radical towards the hydrogen atom (H10) present in the hydroxyl group of 3H3M2B. During this reaction, the reactant O1–H10 has a bond distance of 0.951 Å, from which the H10-atom breaks and subsequently binds with the O3-atom with bond distance of 1.179 Å in the transition state (TS1), whereas the O3–H10 bond in the product (P1) has the bond distance of 0.957 Å. From Table 2, the thermodynamic properties of the reaction indicate that the product (P1) is formed exothermically with ΔH = −15.4 kcal mol−1, and is exergonic with ΔG = −13.55 kcal mol−1. Earlier studies have concluded that most of the hydrogen abstraction processes are exothermic in nature.37–39
O group. This bond has a length of 1.982 Å, while the hydrogen bond formed between the OH radical and the methyl group has a bond distance of 2.658 Å. The reactant complex (RC) proceeds toward the product (PC1) through the transition state (TS1) with an energy barrier of 4.13 kcal mol−1. The product complex (PC1) is formed between the oxygen atom of the carbonyl group and the water molecule with a bond distance of 1.925 Å. Subsequently, the product complex breaks into product (P1) and a water molecule due to the high electron affinity of the oxygen atom (O3) in the hydroxyl radical towards the hydrogen atom (H10) present in the hydroxyl group of 3H3M2B. During this reaction, the reactant O1–H10 has a bond distance of 0.951 Å, from which the H10-atom breaks and subsequently binds with the O3-atom with bond distance of 1.179 Å in the transition state (TS1), whereas the O3–H10 bond in the product (P1) has the bond distance of 0.957 Å. From Table 2, the thermodynamic properties of the reaction indicate that the product (P1) is formed exothermically with ΔH = −15.4 kcal mol−1, and is exergonic with ΔG = −13.55 kcal mol−1. Earlier studies have concluded that most of the hydrogen abstraction processes are exothermic in nature.37–39Here, the product complex (PC3) includes an intermolecular bond between the oxygen atom of the carbonyl group and the hydroxyl group of 3H3M2B with a bond distance of 1.974 Å. In addition intermolecular hydrogen bond exist between hydrogen atom of the methyl group and water molecule with a bond distance of 2.465 Å. Similar to the previous hydrogen abstraction in pathway 2, here the C5–H5 bond from the methyl group breaks and leads to the product P3 along with a water molecule. Here, the reactant complex (RC) is converted into the product complex (PC3) via transition state (TS3) with a small energy barrier of 1.59 kcal mol−1. Like P1 and P2, the pathway to the product (P3) is exothermic with an enthalpy of ΔH = −19.79 kcal mol−1 and a Gibbs free energy ΔG of −19.96 kcal mol−1 respectively.
The obtained three pathways are analogous to most VOC OH-initiated oxidation schemes, the hydrogen abstraction pathways are generally exothermic in nature.36 The energy profiles, corresponding to the three hydrogen abstractions pathways are shown in Fig. 4.
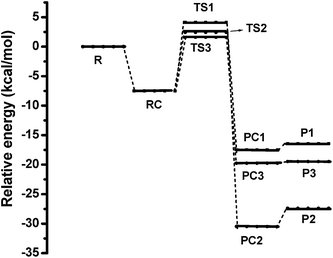 | ||
| Fig. 4 Relative energy profile corresponding to the hydrogen abstraction reaction calculated at BH&HLYP level of theory with 6-311+G(d,p) basis set. | ||
The individual rate constants for all the three pathways were evaluated using the canonical variational transition state theory CVT/SCT over the temperature range of 278 to 1000 K at BH&HLYP level of theory with 6-311+G(d,p) basis set. The calculated rate constant values are presented in Table 3. The theoretical global rate constant value at 298 K is 10.49 × 10−13 cm3 per molecule per s, which is comparable with our experimental value ((4.8 ± 0.8) × 10−13 cm3 per molecule per s). We observe from Table 3, that the most favourable pathway (pathway (3)) has a forward rate constant value of 5.53 × 10−13 cm3 per molecule per s at 298 K. The reverse rate constant has a value of 6.32 × 10−22 cm3 per molecule per s at 298 K for this channel. The equilibrium constant Kc of this hydrogen abstraction reaction is 8.75 × 108 with a transmission coefficient value of 3.82. This value shows the tunneling effect, which plays a major role in the H-atom abstraction process. Likewise, pathway (2) has a rate constant value of 3.32 × 10−13 cm3 per molecule per s and a reverse rate value of 0.60 × 10−27 cm3 per molecule per s at 298 K. The equilibrium rate constant Kc of the reaction is 5.53 × 1014 and the transmission coefficient of 4.28 indicates the tunneling effect of this reaction. From the above analysis, it is noticeable that the forward reaction is more favourable than the reverse one. In addition, the third most favourable pathway (1) has a forward rate constant value of 1.62 × 10−13 cm3 per molecule per s at 298 K with a transmission coefficient of 7.22.
| Temperature (K) | Pathway 1 kI1 (cm3 per molecule per s) | Pathway 2 kI2 (cm3 per molecule per s) | Pathway 3 kI3 (cm3 per molecule per s) | ||
|---|---|---|---|---|---|
| Forward × 10−13 | Forward × 10−13 | Reverse | Forward × 10−13 | Reverse | |
| 278 | 3.13 | 3.62 | 0.41 × 10−28 | 6.34 | 1.53 × 10−22 |
| 288 | 2.23 | 3.46 | 0.16 × 10−27 | 5.91 | 3.19 × 10−22 |
| 298 | 1.62 | 3.32 | 0.60 × 10−27 | 5.53 | 6.32 × 10−22 |
| 308 | 1.21 | 3.20 | 0.20 × 10−26 | 5.21 | 1.19 × 10−21 |
| 318 | 0.91 | 3.08 | 0.62 × 10−26 | 4.92 | 2.17 × 10−21 |
| 328 | 0.70 | 2.98 | 0.18 × 10−25 | 4.67 | 3.81 × 10−21 |
| 338 | 0.55 | 2.88 | 0.49 × 10−25 | 4.45 | 6.46 × 10−21 |
| 348 | 0.43 | 2.79 | 1.26 × 10−25 | 4.25 | 1.06 × 10−20 |
| 350 | 0.41 | 2.77 | 1.51 × 10−25 | 4.21 | 1.16 × 10−20 |
| 450 | 0.0725 | 2.20 | 1.68 × 10−22 | 3.06 | 4.67 × 10−13 |
| 550 | 0.0234 | 1.92 | 1.45 × 10−20 | 2.60 | 4.85 × 10−13 |
| 650 | 0.0106 | 1.77 | 3.21 × 10−19 | 2.39 | 2.46 × 10−13 |
| 750 | 0.0059 | 1.69 | 3.13 × 10−18 | 2.30 | 8.18 × 10−13 |
| 850 | 0.0038 | 1.66 | 1.79 × 10−17 | 2.27 | 2.06 × 10−13 |
| 950 | 0.0026 | 1.64 | 7.19 × 10−17 | 2.28 | 4.33 × 10−13 |
| 1000 | 0.0023 | 1.64 | 1.30 × 10−16 | 2.29 | 5.95 × 10−13 |
The overall rate constants of these three pathways are given in Table 4, these values are determined as the sum of the rate constants of all three reaction pathways. Partial rates to individual exit channels are indicated as well in terms of branching ratios (%).
| Temperature (K) | Overall rate constant × 10−13 | Branching ratios (%) | ||
|---|---|---|---|---|
| Product (P1) | Product (P2) | Product (P3) | ||
| 278 | 13.09 | 23.91 | 27.65 | 48.43 |
| 288 | 11.61 | 19.22 | 29.82 | 50.94 |
| 298 | 10.49 | 15.47 | 31.70 | 52.81 |
| 308 | 9.62 | 12.57 | 33.26 | 54.15 |
| 318 | 8.92 | 10.21 | 34.56 | 55.21 |
| 328 | 8.35 | 8.38 | 35.68 | 55.92 |
| 338 | 7.88 | 6.97 | 36.54 | 56.47 |
| 348 | 7.48 | 5.75 | 37.34 | 56.89 |
| 350 | 7.41 | 5.54 | 37.48 | 56.96 |
| 450 | 5.33 | 1.35 | 41.25 | 57.38 |
| 550 | 4.54 | 0.51 | 42.25 | 57.22 |
| 650 | 4.17 | 0.25 | 42.43 | 57.30 |
| 750 | 3.99 | 0.14 | 42.29 | 57.55 |
| 850 | 3.93 | 0.09 | 42.19 | 57.70 |
| 950 | 3.92 | 0.06 | 41.80 | 58.12 |
| 1000 | 3.93 | 0.05 | 41.70 | 58.23 |
4.3 Branching ratios
The branching ratios (BR) for the corresponding hydrogen abstraction pathways calculated at BH& HLYP level of theory with 6-311+G(d,p) basis set are listed in Table 4 over the temperature range of 278 to 1000 K. It is noticeable that the largest BR at 298 K is for pathway (3) (52.81%) and provides significant contribution towards the reaction of 3H3M2B with OH radical when compared with pathways (1) and (2), whose values are 15.47% and 31.70%, respectively. Thus, from the above-mentioned analysis it is evident that pathway (3) has a larger contribution than pathways (1) and (2) due to a smaller barrier height. Therefore, the hydrogen abstraction seems to occur mainly from one of the methyl groups adjacent to the hydroxyl group of 3H3M2B. From Table 4, we see that the branching ratio values of pathways (2) and (3) increase with the increase in the temperature except above 750 K for pathway (2).4.4 Temperature dependence – Arrhenius parameters
A slight negative temperature dependence was observed over the temperature range explored in this work for theoretical data, whereas, no significant effect of the temperature on the reactivity was observed in our experimental results. The latter were well fitted over the range (277–353 K) by using a standard Arrhenius equation and the following expression was obtained: k(T)exp = (1.88 ± 0.47) × 10−13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp((281 ± 77)/T) in cm3 per molecule per s. The theoretical values, on the other hand, appeared to be better fitted by a modified Arrhenius expression:
exp((281 ± 77)/T) in cm3 per molecule per s. The theoretical values, on the other hand, appeared to be better fitted by a modified Arrhenius expression:  in the range (278–1000 K) than using the conventional Arrhenius formula. Concerning partial rate constants, the temperature dependence is shown in Fig. 5a and b for the calculated rate constants and the predicted ones using the following expressions, taking into account the tunnelling correction (in cm3 per molecule per s):
in the range (278–1000 K) than using the conventional Arrhenius formula. Concerning partial rate constants, the temperature dependence is shown in Fig. 5a and b for the calculated rate constants and the predicted ones using the following expressions, taking into account the tunnelling correction (in cm3 per molecule per s):Pathway (1): k1(T) = (4.0 ± 1.0) × 10−17(T/300)−0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×exp((2483 ± 70)/T) ×exp((2483 ± 70)/T) |
Pathway (2): k2(T) = (0.64 ± 0.03) × 10−13(T/300)0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×exp((489 ± 18)/T) ×exp((489 ± 18)/T) |
Pathway (3): k3(T) = (0.32 ± 0.01) × 10−13(T/300)0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×exp((848 ± 11)/T) ×exp((848 ± 11)/T) |
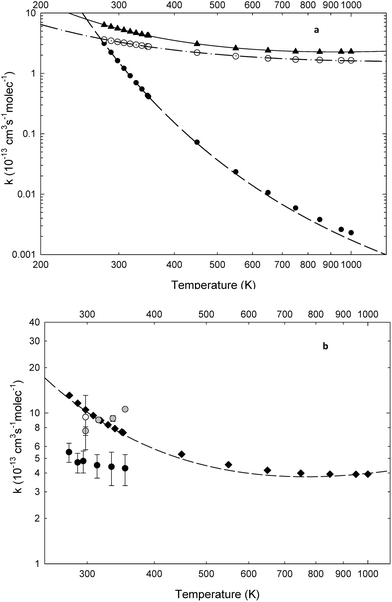 | ||
Fig. 5 Theoretical and experimental rate constants for the reaction of 3H3M2B with OH as a function of temperature. Partial and global rate constants obtained in this work are fitted according to the temperature dependence expressions given in Section 4.4. (a) Partial theoretical rate constants obtained in this work;  pathway 1; pathway 1;  pathway 2; pathway 2;  pathway 3; (b) global rate constants obtained in this work and in the literature: pathway 3; (b) global rate constants obtained in this work and in the literature:  global theoretical rate constants obtained in this work; global theoretical rate constants obtained in this work;  experimental rate constants obtained in this work, experimental rate constants obtained in this work,  experimental rate constant obtained by Aschmann et al. 2000 (ref. 9), experimental rate constant obtained by Aschmann et al. 2000 (ref. 9),  experimental rate constants obtained by Bouzidi et al. 2014 (ref. 13). experimental rate constants obtained by Bouzidi et al. 2014 (ref. 13). | ||
The following temperature dependence k(T)theory = (0.17 ± 0.03) × 10−13 (T/300)1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp((1226 ± 55)/T) in cm3 per molecule per s was obtained for the overall theoretical rate constants. We insist that these expressions, k(T)exp and k(T)theory, are only valid over the studied temperature range. The parameters obtained from these expressions are not intended to be physically meaningful but rather to provide an easy way to introduce our values results in models of atmospheric chemistry with a good level of confidence. A modified Arrhenius adjustment was also applied to our experimental results leading to a fit as good as for the Arrhenius expression mentioned above. However, due to the reduced temperature range, this adjustment lead to a temperature power law subject to large errors even for small extrapolations of temperature outside the temperature range explored in our work.
exp((1226 ± 55)/T) in cm3 per molecule per s was obtained for the overall theoretical rate constants. We insist that these expressions, k(T)exp and k(T)theory, are only valid over the studied temperature range. The parameters obtained from these expressions are not intended to be physically meaningful but rather to provide an easy way to introduce our values results in models of atmospheric chemistry with a good level of confidence. A modified Arrhenius adjustment was also applied to our experimental results leading to a fit as good as for the Arrhenius expression mentioned above. However, due to the reduced temperature range, this adjustment lead to a temperature power law subject to large errors even for small extrapolations of temperature outside the temperature range explored in our work.
Among the three studied pathways, pathway (1) seems to significantly depend on the temperature as seen in Table 4 and Fig. 5a. In fact, the branching ratio and the rate constant values for this pathway increases significantly with decreasing temperature for the whole temperature range (278–1000 K), suggesting that at lower temperatures this channel may dominate the kinetic process. In Table 1, predicted overall values obtained using the modified Arrhenius expression k(T)theory cited above are shown for comparison with experimental ones at the studied temperatures.
4.5 Comparison with previous studies
The reaction of 3H3M2B with OH has been the subject of two previous studies carried out by Aschmann et al.9 and very recently by Bouzidi et al.13 In their study, these authors investigated the kinetics of this reaction by using a relative rate technique. The overall rate constant of our theoretical results (10.49 × 10−13 cm3 per molecule per s) obtained at 298 K is in very good agreement (10%) with that reported by Aschmann et al.9 ((9.4 ± 3.7) × 10−13 cm3 per molecule per s) at 298 K and 27% higher than that reported by Bouzidi et al.13 ((7.6 ± 0.5) × 10−13 cm3 per molecule per s) at 298 K. Our absolute rate constant obtained experimentally at room temperature is lower by a factor of 1.9 than that obtained by Aschmann et al.9 obtained at atmospheric pressure, however an overlap was found between both values taking into account the uncertainties. This discrepancy could be partially due to the “slightly high” rate constant of the reference reaction of n-octane with OH (kn-octane), used in the study carried out by Aschmann et al.9 where a value of (8.67 ± 1.73) cm3 per molecule per s reported by Atkinson et al.40 was used. Indeed, more recent and lower values for (kn-octane) of 8.07 × 10−12, (7.2 ± 1.2) × 10−12 and (8.3 ± 1.6) × 10−12 cm3 per molecule per s have been reported by Atkinson et al.,41 Wilson et al.42 and Li et al.,43 respectively. Despite the observed scattering in the literature, a median value of about (8 ± 2) × 10−12 cm3 per molecule per s encompasses all the previous mentioned investigations of kn-octane at 294 K and yields a corrected value of 8.7 × 10−13 cm3 per molecule per s for the rate constant of the reaction of 3H3M2B with OH obtained by Aschmann et al.9 Very recently, Bouzidi et al.,13 studied the reaction of 3H3M2B with OH over the temperature range of 298 to 356 K by using a relative technique at 500–600 Torr in purified air. The value reported by these authors ((7.6 ± 0.5) × 10−13 cm3 per molecule per s) at 298 K is higher by a factor of 1.5 than our experimental value and lower by a factor of 1.2 than that reported by Aschmann et al.9 In their study, Bouzidi et al.13 used tert-butyl alcohol as the reference compound and a rate constant of 10.8 × 10−13 cm3 per molecule per s reported by Téton et al.44 for the reference reaction. However, other values of the rate constant of the reference reaction are available in the literature (cm3 per molecule per s) as follows: (11.1 ± 0.7),45 (8.1 ± 1.7) (ref. 46) and (10.7 ± 0.8).47 A median value of about (9 ± 3) × 10−13 cm3 per molecule per s encompasses all the previous mentioned values of ktert-butyl alcohol at room temperature and yields a corrected value of 6.33 × 10−13 cm3 per molecule per s for that obtained by Bouzidi et al.13As mentioned before, the reaction of 3H3M2B with OH was studied as a function of temperature in the present study over the range 277–353 K and by Bouzidi et al.13 over the range 298–356 K, respectively. In both studies, no significant effect of the temperature on the reactivity of 3H3M2B with OH was observed.
Based on reported previous studies9,10 the reaction of aliphatic ketones with OH is expected to proceed by H abstraction from the C–H bonds. However, the kinetics of 3H3M2B with OH studied in this work and reported in the literature was found slower than other α-hydroxyketones investigated by Aschmann et al.9 and Messaadia et al.10 In fact, the following rate constants (in cm3 per molecule per s) have been reported in these studies: (7.7 ± 0.7) × 10−12, (9.60 ± 0.30) × 10−12 and (15.1 ± 3.1) × 10−12 for 1-hydroxy-2-butanone,9 3-hydroxy-2-butanone10 and 4-hydroxy-3-hexanone,9 respectively. This “out of trend” decrease in reactivity with OH observed for 3H3M2B may be explained by the lack of C–H bonds on the tertiary carbon atom, activated by the OH group in the 3H3M2B structure.
4.6 Atmospheric lifetime
The rate constant determined in this work is used to calculate the tropospheric lifetime of 3H3M2B due to its reaction with OH radicals according to the following relation: τ = 1/k[OH] where [OH] is the average concentration of OH and k the rate constant for the reaction of 3H3M2B with OH reported in this work at room temperature. Values of 24 and 11 days were obtained from our experimental and theoretical values, respectively and by using a 24 h daytime average global tropospheric OH radical concentration of 1 × 106 molecule per cm3.40 The reaction of 3H3M2B with Cl atoms has been recently investigated12 and a lifetime of 102 days was obtained by using an average global tropospheric concentration of Cl atoms of 1 × 103 molecule per cm3.48 The reactivity of 3H3M2B towards NO3 radicals and O3 has been investigated by Aschmann et al.9 and lower limits of 230 days and 150 days were found for lifetimes due to NO3 and O3, respectively. Bouzidi et al.13 investigated the photolysis process of 3H3M2B and reported a relatively short atmospheric lifetime of 4–5 days. In the light of the obtained results, it is obvious that the reaction of 3H3M2B with OH is a significant removal chemical process for this species in the atmosphere in addition to photolysis. Given the short tropospheric lifetime, this reaction could have a role in the chemistry of the troposphere at local scale.Acknowledgements
The authors gratefully thank the INSU-LEFE French programme and Brittany Regional Council for financial support. The authors would like to thank Dr Dahbia Talbi and Dr Yohann Scribano – University of Montpellier 2-France for their fruitful discussions.References
- R. Atkinson, J. Phys. Chem. Ref. Data, Monogr., 1989, 1, 1 Search PubMed.
- R. Atkinson, J. Phys. Chem. Ref. Data, Monogr., 1994, 2, 1 CAS.
- N. Sebbar, J. W. Bozzelli and H. Bockhorn, J. Phys. Chem. A, 2013, 118, 21–37 CrossRef PubMed.
- J. M. Hudzik and J. W. Bozzelli, J. Phys. Chem. A, 2012, 116, 5707–5722 CrossRef CAS PubMed.
- C. Schulz and V. Sick, Prog. Energy Combust. Sci., 2005, 31, 75–121 CrossRef CAS.
- R. Hanson, J. Seitzman and P. Paul, Appl. Phys. B, 1990, 50, 441–454 CrossRef.
- P. Pepiot-Desjardins, H. Pitsch, R. Malhotra, S. R. Kirby and A. L. Boehman, Combust. Flame, 2008, 154, 191–205 CrossRef CAS.
- Z. Hong, D. F. Davidson, S. S. Vasu and R. K. Hanson, Fuel, 2009, 88, 1901–1906 CrossRef CAS.
- S. M. Aschmann, J. Arey and R. Atkinson, J. Phys. Chem. A, 2000, 104, 3998–4003 CrossRef CAS.
- L. Messaadia, G. El Dib, M. Lendar, M. Cazaunau, E. Roth, A. Ferhati, A. Mellouki and A. Chakir, Atmos. Environ., 2013, 77, 951–958 CrossRef CAS.
- G. El Dib, C. Sleiman, A. Canosa, D. Travers, J. Courbe, T. Sawaya, I. Mokbel and A. Chakir, J. Phys. Chem. A, 2013, 117, 117–125 CrossRef PubMed.
- C. Sleiman, G. El Dib, B. Ballesteros, A. Moreno, J. Albaladejo, A. Canosa and A. Chakir, J. Phys. Chem. A, 2014, 118, 6163–6170 CrossRef CAS PubMed.
- H. Bouzidi, H. Laversin, A. Tomas, P. Coddeville, C. Fittschen, G. El Dib, E. Roth and A. Chakir, Atmos. Environ., 2014, 98, 540–548 CrossRef CAS.
- L. Messaadia, G. El Dib, A. Ferhati, E. Roth and A. Chakir, Chem. Phys. Lett., 2012, 529, 16–22 CrossRef CAS.
- W. B. DeMore, S. P. Sander, D. M. Golden, R. F. Hampson, M. J. Kurylo, C. J. Howard, A. R. Ravishankara, C. E. Kolb and M. J. Molina, JPL Publ., 1997, 97 Search PubMed.
- W. Kohn, A. D. Becke and R. G. Parr, J. Phys. Chem., 1996, 100, 12974–12980 CrossRef CAS.
- Y. Sun, Q. Zhang, J. Hu, J. Chen and W. Wang, Chemosphere, 2015, 119, 626–633 CrossRef CAS PubMed.
- C. Xu and L. Wang, J. Phys. Chem. A, 2013, 117, 2358–2364 CrossRef CAS PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 1372–1377 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS.
- C. Gonzalez and H. B. Schlegel, J. Chem. Phys., 1989, 90, 2154–2161 CrossRef CAS.
- C. Gonzalez and H. B. Schlegel, J. Phys. Chem., 1990, 94, 5523–5527 CrossRef CAS.
- A.-Y. Yu, Struct. Chem., 2014, 25, 607–615 CrossRef CAS.
- G. C. Song, X. J. Jia, Y. Gao, J. Luo, Y. B. Yu, R. S. Wang and X. M. Pan, J. Phys. Chem. A, 2010, 114, 9057–9068 CrossRef CAS PubMed.
- B. Chan and L. Radom, J. Phys. Chem. A, 2012, 116, 3745–3752 CrossRef CAS PubMed.
- B. J. Lynch, P. L. Fast, M. Harris and D. G. Truhlar, J. Phys. Chem. A, 2000, 104, 4811–4815 CrossRef CAS.
- C. Møller and M. S. Plesset, Phys. Rev., 1934, 46, 618–622 CrossRef.
- M. J. Frisch, M. Head-Gordon and J. A. Pople, Chem. Phys. Lett., 1990, 166, 281–289 CrossRef CAS.
- A. Fernandez-Ramos, J. A. Miller, S. J. Klippenstein and D. G. Truhlar, Chem. Rev., 2006, 106, 4518–4584 CrossRef CAS PubMed.
- B. C. Garrett and D. G. Truhlar, J. Am. Chem. Soc, 1979, 101, 4534–4548 CrossRef CAS.
- B. C. Garrett and D. G. Truhlar, J. Chem. Phys., 1979, 70, 1593–1598 CrossRef CAS.
- B. C. Garrett, D. G. Truhlar, R. S. Grev and A. W. Magnuson, J. Phys. Chem., 1980, 84, 1730–1748 CrossRef CAS.
- S. W. Benson, Thermochemical kinetics, Wiley&Sons, New York, 1976 Search PubMed.
- J. Zheng, S. Zhang, J. C. Corchado, Y. Y. Chuang, E. L. Coitino, B. A. Ellingson and D. G. Truhlar, GAUSSRATE Version, 2009 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, GAUSSIAN, Inc., Wallingford, CT, 2009.
- J. Zheng, S. Zhang, B. J. Lynch, J. C. Corchado, Y. Y. Chaung, P. L. Fast, W. P. Hu, Y. P. Liu, G. C. Lynch, K. A. Nguyen, C. F. Jackels, A. F. Ramos, B. A. Ellingson, V. S. Melissas, J. Villa, I. Rossi, E. L. Coitino, J. Pu and T. V. Albu, POLYRATE VERSION, 2010 Search PubMed.
- S. B. Barone, A. A. Turnipseed and A. R. Ravishankara, J. Phys. Chem., 1996, 100, 14694–14702 CrossRef CAS.
- C. Zavala-Oseguera and A. Galano, J. Chem. Theory Comput., 2009, 5, 1295–1303 CrossRef CAS.
- A. M. Priya and L. Senthilkumar, RSC Adv., 2014, 4, 23464–23475 RSC.
- R. Atkinson, D. L. Baulch, R. A. Cox, R. F. Hampson, J. A. Kerr, M. J. Rossi and J. Troe, J. Phys. Chem. Ref. Data, 1997, 26, 1329–1499 CrossRef CAS.
- R. Atkinson, Atmos. Chem. Phys., 2003, 3, 2233–2307 CrossRef CAS.
- E. W. Wilson, W. A. Hamilton, H. R. Kennington, B. Evans, N. W. Scott and W. B. DeMore, J. Phys. Chem. A, 2006, 110, 3593–3604 CrossRef CAS PubMed.
- Z. Li, S. Singh, W. Woodward and L. Dang, J. Phys. Chem. A, 2006, 110, 12150–12157 CrossRef CAS PubMed.
- S. Téton, A. Mellouki, G. Le Bras and H. Sidebottom, Int. J. Chem. Kinet., 1996, 28, 291–297 CrossRef.
- H. Wu, Y. Mu, X. Zhang and G. Jiang, Int. J. Chem. Kinet., 2003, 35, 81–87 CrossRef CAS.
- S. M. Saunders, D. L. Baulch, K. M. Cooke and M. J. Pilling, Int. J. Chem. Kinet., 1994, 26, 113–130 CrossRef CAS.
- T. J. Wallington, P. Dagaut, R. Liu and M. J. Kurylo, Environ. Sci. Technol., 1988, 22, 842–844 CrossRef CAS PubMed.
- O. W. Wingenter, D. R. Blake, N. J. Blake, B. C. Sive, F. S. Rowland, E. Atlas and F. Flocke, J. Geophys. Res.: Atmos., 1999, 104, 21819–21828 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1 optimized reactive species of all reactants, reactant complex, transition states, products, product complexes at BH & HLYP level of theory with 6-311+G(d,p) basis set. Bond distances are in angstrom, bond angles are in degrees. See DOI: 10.1039/c4ra15664a |
| This journal is © The Royal Society of Chemistry 2015 |

