A highly selective and sensitive turn-on fluorescent probe for the detection of holmium ion and its bioimaging†
Yanqing Guoa,
Fangjun Huob,
Caixia Yin*c,
Jin Kangc and
JianFang Lib
aCollege of Chemistry and Chemical Engineering, Jinzhong University, Jinzhong 030600, China
bResearch Institute of Applied Chemistry, Shanxi University, Taiyuan, 030006, China
cInstitute of Molecular Science, Shanxi University, Taiyuan 030006, China. E-mail: yincx@sxu.edu.cn; Fax: +86 351 7011022; Tel: +86 351 7011022
First published on 15th December 2014
Abstract
Phenolphthalein aldehyde was synthesized and used as a turn-on fluorescent probe for the detection of holmium ion (Ho3+), which is one of the lanthanide ions, in HEPES–DMF = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v, pH = 7.4) solution with an excellent selectivity and sensitivity for Ho3+ over other metal ions.
1 (v/v, pH = 7.4) solution with an excellent selectivity and sensitivity for Ho3+ over other metal ions.
Rare earth (RE) metals are important non-renewable resources. With the development of science, there have been various demands for materials. Application of rare earth elements, due to their homogeneous properties, extends to the use of single rare earth specialties and has gone deep into all areas of modern technology. Because rare earth elements (REE) have special properties to promote crop production and improve crop quality, the kapplication of compound fertilizer of rare earth elements is more and more wide spread. Generally, low concentrations of REEs are present in soil, plant, water, and atmosphere, but REEs can accumulate in such environments following anthropogenic inputs because of the low mobility of these elements.1–3 As the products containing rare earth elements continuously enter into the environment, long-term risks to plants, animals, the environment and human health has attracted widespread attention at home and abroad in the scientific community.
Holmium is one of lanthanide ions, and it is 20 times more abundant than silver.4 There is a growing trend in the uses of holmium, due to the fact that it is studied to produce catalysts, polish glass and used for biological applications.5–7 Lanthanides do not partake in any metabolic process. However, due to the similarity of the ionic radii of lanthanides to calcium ion, possessing a higher charge, they exhibit a high affinity for the Ca2+ sites on biological molecules and a stronger binding to water molecules.7–10 The bioaccumulation of the holmium ions in the body can be a threat to the liver. Nowadays, it is known that holmium causes damage to the cell membranes of water animals with negative effects on the reproduction and the nervous systems.7,8,10–12 This makes the detections of Ho greatly important. Conventional methods of Ho ion analysis have included neutron activation analysis, ICP-MS, ICP-AES and isotope dilution mass spectrometry.13–18 In recent years, a number of opcode and membrane sensors for selective determination of Ho3+ (ref. 4, 10, 18–25) have been reported. However, a fluorescent probe to detect Ho3+ has not been reported.
Herein, we report a novel fluorescent probe based on phenolphthalein aldehyde to selectively detect Ho3+ in a medium of near neutral pH. In addition, the recognition ability of the probe for Ho3+ was studied. The result showed that this probe works well at physiological pH and has a high selectivity and sensitivity for Ho3+ over other metal ions. These desirable attributes render the sensor suitable for the detection of Ho3+. Furthermore, the system is used for bioimaging.
The synthesis of probe is summarized in Scheme 1. To a stirred TFA (10 mL) on ice was added phenolphthalein (636.64 mg, 2 mmol) and hexamethylenetetraamine (403.7 mg, 2.88 mmol). The solution was allowed to warm to room temperature, and then it was refluxed for 8 h. The excess TFA was removed under vacuum. 30 mL water was then added to the remaining solution, and the mixture was warmed at 60 °C whilst stirring for another 30 min. Upon cooling on ice, a pale-yellow solid was precipitated out from the solution, which was collected by filtration. Then the crude product was purified by chromatography on a silica gel column, and give the desired product as a white solid (57% yield). 1H NMR (300 MHz, 25 °C, DMSO-d6): δ 11.04 (bs, 2H), 10.22 (bs, 2H), 7.94 (d, 1H, J = 7.2 Hz), 7.84 (m, 2H, J = 20.7 Hz), 7.68 (q, 1H, J = 14.4 Hz), 7.51 (s, 2H), 7.43 (d, 2H, J = 8.7 Hz), 7.02 (d, 2H, J = 8.7 Hz); 13C NMR (75 MHz, DMSO-d6): δ 189.9, 168.1, 160.3, 150.5, 134.6, 134.2, 130.7, 129.4, 125.7, 125.1, 123.6, 121.1, 117.5, 89.3; ESI-MS [M − H]− 373.25; elemental analysis (calcd%) for C22H14O6: C, 70.59; H, 3.77; found: C, 70.38; H, 3.89 (Fig. S1, ESI†).
The recognition ability of the probe was investigated by fluorescence spectroscopy. The selectivity towards metal ions is one of the most important criteria for probe design. The effect of a wide range of environmentally and physiologically active metal ions was investigated for the probe using the fluorescence spectra of solutions containing probe and the metal ions (100 equiv.) in the HEPES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF = 1
DMF = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
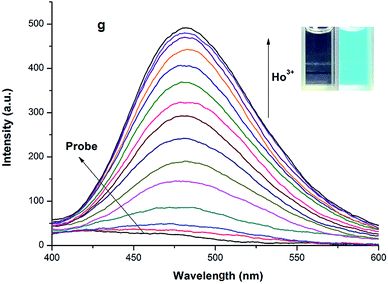
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v pH = 7.4) solution. As shown in Fig. 1, although metal ions, such as Cu2+, Cr2+, Gd3+, Ni2+, Co2+, VO2+, La3+, Pb2+, Zr4+, Mn2+, Zn2+, Ru3+, Yb3+, Cd2, Sm3+, Bi3+, Ce4+, Er3+, Ca2+, Mg2+, Sn2+, Fe3+, Dy3+, Pr3+, Tb3+, Pm3+, Lu3+, Tm3+, Nd3+ and Eu3+, do not result in any apparent changes in fluorescence intensity, there is notable change when Ho3+ is involved (λex = 365 nm) (Fig. S2, ESI†). Fig. 1 shows that fluorescence optical density at 480 nm when various metal ions are added and fluorescence color changes.
1 (v/v pH = 7.4) solution. As shown in Fig. 1, although metal ions, such as Cu2+, Cr2+, Gd3+, Ni2+, Co2+, VO2+, La3+, Pb2+, Zr4+, Mn2+, Zn2+, Ru3+, Yb3+, Cd2, Sm3+, Bi3+, Ce4+, Er3+, Ca2+, Mg2+, Sn2+, Fe3+, Dy3+, Pr3+, Tb3+, Pm3+, Lu3+, Tm3+, Nd3+ and Eu3+, do not result in any apparent changes in fluorescence intensity, there is notable change when Ho3+ is involved (λex = 365 nm) (Fig. S2, ESI†). Fig. 1 shows that fluorescence optical density at 480 nm when various metal ions are added and fluorescence color changes.
How the probe could specifically recognize Ho3+ was explained as following: the peripheral electron shell configuration of Ho3+ is 4f10, which results in abundant level structures as well as some high metastable states. The efficient isolation between energy levels prompts Ho3+ to have abundant electron transition.26 Its peculiar energy level structure inducing the bigger gap between 3k8, 5s2 and the adjacent low level makes non-radiative relaxation to not occur easily. Furthermore, the absorption peak of Ho3+ is very rich, which resulted from two reasons: one is that a low energy photon is easy to excite Ho3+ ions, another is other rare earth elements with sensitized effect realizes inversion of high level particle number to obtain the strong blue-green glow.27,28 Green glow corresponds to the energy level transition of 5S2(5F4)–5I8 of the Ho3+ spectral term. The fluorescence of Dy3+ is easily quenched by high-energy vibration of O–H bonds in water molecules.29
A titration experiment of the probe for Ho3+ was also carried out using a fluorescence spectrophotometer. The probe displayed no emission at 480 nm. With addition and gradual increasing the amount of Ho3+ to the HEPES–DMF = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v, pH = 7.4) solution containing probe, a significant increase in fluorescence intensity at 480 nm was observed with an excitation wavelength of 365 nm. When 260 μM Ho3+ was added to the solution of the probe (20 μM), a more than 25-fold increase in fluorescence intensity at 526 nm was observed (Fig. 2) with a change of fluorescence quantum yield from φprobe = 0.001 to φprobe-Ho3+ = 0.256. In addition, with increasing Cu2+ concentration, the absorption peak at 330 gradually decreased and the absorption peak at 380 nm increased. One isosbestic point was noted at 348 nm, indicating the formation of a new species (Fig. S3, ESI†). According to linear Benesi Hildebrand expression, the measured intensity [1/(A − A0)] at 380 nm varied as a function of 1/[Ho3+] in a linear relationship (R = 0.99797) (Fig. S4, ESI†), which indicates the formation of 1
1 (v/v, pH = 7.4) solution containing probe, a significant increase in fluorescence intensity at 480 nm was observed with an excitation wavelength of 365 nm. When 260 μM Ho3+ was added to the solution of the probe (20 μM), a more than 25-fold increase in fluorescence intensity at 526 nm was observed (Fig. 2) with a change of fluorescence quantum yield from φprobe = 0.001 to φprobe-Ho3+ = 0.256. In addition, with increasing Cu2+ concentration, the absorption peak at 330 gradually decreased and the absorption peak at 380 nm increased. One isosbestic point was noted at 348 nm, indicating the formation of a new species (Fig. S3, ESI†). According to linear Benesi Hildebrand expression, the measured intensity [1/(A − A0)] at 380 nm varied as a function of 1/[Ho3+] in a linear relationship (R = 0.99797) (Fig. S4, ESI†), which indicates the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between Ho3+ and probe. The association constant of probe with Ho3+ was calculated to be 1.08 × 107 M−1.
1 stoichiometry between Ho3+ and probe. The association constant of probe with Ho3+ was calculated to be 1.08 × 107 M−1.
The effect of pH on the fluorescence properties of the system was investigated for pH values of 2.0–13.0. The results are shown in Fig. 3. Over the pH 2.0–8.0, the free probe exhibits no fluorescence in the HEPES–DMF = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v, pH = 7.4) solution. It is evident that the fluorescence signal of the probe increases significantly with increasing pH from 8.0 to 12.0. However, the intensity of the probe exhibits a non-ignorable decrease at pH 13.0. Moreover, the fluorescence signal for the probe solution containing Ho3+ increased sharply from pH 4.0 to 8.0, and then decreased from pH 8.0 to 11.0. The fluorescence intensity of the probe solution containing Ho3+ slightly increases at pH 12.0 compared with at pH 11.0. Holmium exists in the form of Ho(OH)3 precipitate due to the strong basicity of pH 13.0, which results in the fluorescence decrease of probe with the addition of Ho3+. Therefore, considering pH and fluorescence intensity, a pH of 7.4 was selected for the analytical system.
1 (v/v, pH = 7.4) solution. It is evident that the fluorescence signal of the probe increases significantly with increasing pH from 8.0 to 12.0. However, the intensity of the probe exhibits a non-ignorable decrease at pH 13.0. Moreover, the fluorescence signal for the probe solution containing Ho3+ increased sharply from pH 4.0 to 8.0, and then decreased from pH 8.0 to 11.0. The fluorescence intensity of the probe solution containing Ho3+ slightly increases at pH 12.0 compared with at pH 11.0. Holmium exists in the form of Ho(OH)3 precipitate due to the strong basicity of pH 13.0, which results in the fluorescence decrease of probe with the addition of Ho3+. Therefore, considering pH and fluorescence intensity, a pH of 7.4 was selected for the analytical system.
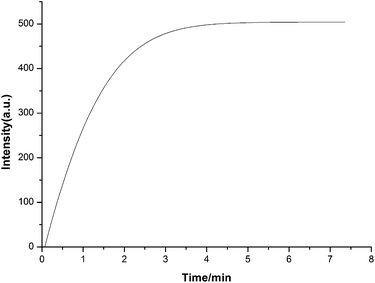
The effect of reaction time on the fluorescence emission of the system was also studied in the presence of 10 equiv. of Ho3+ and the results are shown in Fig. 4. The fluorescence signal for the system increases gradually with increasing reaction time, and the addition of Ho3+, under the selected reaction conditions, was rapid. Therefore, a 4 min reaction time was selected for the following experiments.
To investigate the detection limit of probe for Ho3+, probe (20 μM) was treated with various concentrations of Ho3+ (0–260 μM) and the relative emission intensities at 480 nm were plotted as a function of the Ho3+ concentration (Fig. S5, ESI†). The fluorescence intensity of probe is linearly proportional to the Ho3+ concentrations, and the detection limit, based on the definition by IUPAC (CDL = 3 Sb m−1),30 was found to be 0.055 μM, indicating that this probe is sensitive enough to monitor Ho3+ compared with other methods (Table 1).18,19,23,25
The reaction mechanism of the present system was also studied. We presumed that the fluorescence increase could be attributed to ring-open after the coordination of probe with Ho3+. Hydroxyl and aldehyde groups (their O atoms as hard bases) of the probe easily coordinating with Ln ions as hard acids and resulting in the ring open, increased the conjugate structure of intramolecular, which lead to the system became strong fluorescence. The identification of the coordination product in the ESI-MS analysis made it possible to propose the signaling mechanism: a peak at m/z = 984.00, corresponding to [probe-Ho + 4H2O + H]+, is clearly observed (Fig. S6, ESI†). The proposed detection mechanism and the structures of the probes, both with and without the addition of Ho3+, are shown in Scheme 2.
The ability of probe to react with Ho3+ within living cells was also evaluated by laser confocal fluorescence imaging using a Leica TCS SP5 laser scanning microscope. The optical window at the yellow channel (450–550 nm) was chosen as a signal output. As shown in Fig. 5a, HepG2 cells that were incubated with 20 μM probe for 30 min at 37 °C and washed 3 times with PBS showed no fluorescence. In a further experiment it was found that HepG2 cells displayed green fluorescence when the cells were first incubated with 20 μM of probe for 30 min at 37 °C and washed 3 times with PBS, then incubated with 100 μM HoCl3 and washed 3 times with PBS (Fig. 5b). These cell experiments show the good cell-membrane permeability of probe, and it can thus be used to mark Ho3+ within living cells.
In summary, we have developed a fluorescent probe for the detection of Ho3+ by turn-on fluorescence over other analytes in aqueous solution. This probe is based on a phenolphthalein derivative which has a high selectivity and sensitivity for Ho3+ over other analytes in HEPES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF = 1
DMF = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v pH = 7.4). Furthermore, the system is used for bioimaging.
1 (v/v pH = 7.4). Furthermore, the system is used for bioimaging.
The work was supported by the National Natural Science Foundation of China (no. 21102086, 21472118), the Shanxi Province Science Foundation for Youths (nos 2012021009-4 and 2013011011-1), the Shanxi Province Foundation for Returnee (no. 2012-007), the Taiyuan Technology Star Special (no. 12024703), the Program for the Top Young and Middle-aged Innovative Talents of Higher Learning Institutions of Shanxi (TYMIT, no. 2013802), Talents Support Program of Shanxi Province (no. 2014401) and CAS Key Laboratory of Analytical Chemistry for Living Biosystems Open Foundation (no. ACL201304).
Notes and references
- X. D. Cao, X. R. Wang and G. W. Zhao, Chemosphere, 2000, 40, 23–28 CrossRef CAS.
- L. D. Aquino, M. Morgana, M. A. Carboni, M. Staiano, M. V. Antisari, M. Re, M. Lorito, F. Vinale, K. M. Abadi and S. L. Woo, Soil Biol. Biochem., 2009, 41, 2406–2413 CrossRef PubMed.
- S. Zhang and X. Shan, Environ. Pollut., 2001, 112, 395–405 CrossRef CAS.
- H. A. Zamani, A. Zanganeh-Asadabadi, M. Rohani, M. S. Zabihi, J. Fadaee, M. R. Ganjalic, F. Faridbodc and S. Meghdadi, Mater. Sci. Eng., C, 2013, 33, 984–988 CrossRef CAS PubMed.
- J. Kabalin, P. Gilling and M. Fraundorfer, J. Clin. Laser Med. Surg., 1998, 16, 21–27 CAS.
- O. R. Kirk and F. D. Othmer, Encyclopedia of Chemical Technology, Wiley, New York, 1982, vol. 19, p. 851 Search PubMed.
- V. S. Sastri, J. C. G. Bunzli, V. R. Rao, G. V. S. Rayudu and J. R. Perumareddi, Modern Aspects of Rare Earths and Their Complexes, Elsevier publication, Elsevier, Amsterdam, 2003 Search PubMed.
- S. P. Fricker, Chem. Soc. Rev., 2006, 35, 524–533 RSC.
- K. Wang, Y. Cheng, X. Yang and R. Li, Met. Ions Biol. Syst., 2003, 40, 707–751 CAS.
- F. Faridbod, M. R. Ganjali, B. Larijani, M. Hosseini and P. Norouzi, Mater. Sci. Eng., C, 2010, 30, 555–560 CrossRef CAS PubMed.
- M. R. Ganjalia, M. Hosseini, A. Karimi, H. Haji-Hashemi, M. Salavati-Niasarid and P. Norouzi, Spectrochim. Acta, Part A, 2014, 121, 224–229 CrossRef PubMed.
- O. Vicente, A. Padró, L. Martinez, R. Olsina and E. Marchevsky, Spectrochim. Acta, Part B, 1998, 53, 1281–1287 CrossRef.
- R. S. Houk, V. A. Fassel, G. D. Reach and H. J. Svec, Anal. Chem., 1980, 52, 2283–2289 CrossRef CAS.
- R. Al-Merey and H. J. M. Bowen, J. Radioanal. Nucl. Chem., 1991, 153, 221–234 CrossRef CAS.
- M. Anbu, T. P. Rao, C. S. P. Iyer and A. D. Damodaran, Anal. Chem., 1996, 41, 781–785 CAS.
- N. X. Wang, W. Jiang, Z. K. Si and Z. Qi, Mikrochim. Acta, 1997, 126, 251–255 CrossRef CAS.
- J. Li, S. Liu, X. Mao, P. Gao and Z. Yan, J. Electroanal. Chem., 2004, 561, 137–142 CrossRef CAS PubMed.
- M. R. Ganjali, H. Shams, F. Faridbod, L. Hajiaghababaei and P. Norouzi, Mater. Sci. Eng., C, 2009, 29, 1380–1386 CrossRef CAS PubMed.
- M. R. Ganjali, R. Nemati, F. Faridbod, P. Norouzi and F. Darviche, Int. J. Electrochem. Sci., 2008, 3, 1288–1298 CAS.
- M. R. Ganjali, P. Norouzi, M. Adib and A. Ahmadalinezhad, Anal. Lett., 2006, 39, 1075–1086 CrossRef CAS.
- M. R. Ganjali, S. Rasoolipour, M. Rezapour, P. Norouzi, M. Amirnasrb and S. Meghdadi, J. Braz. Chem. Soc., 2006, 17, 1211–1216 CAS.
- H. A. Zamani, M. R. Ganjali, P. Norouzi and S. Meghdadi, J. Appl. Electrochem., 2007, 37, 853–859 CrossRef CAS.
- M. R. Ganjali, S. Rasoolipoura, M. Rezapoura, P. Norouzi, M. Amirnasr and S. Meghdadi, Sens. Actuators, B, 2006, 119, 89–93 CrossRef CAS PubMed.
- H. A. Zamani, R. Fatemeh, N. R. Fatemeh, A. Ali, I. Alihossien, R. G. Mohammad, F. Farnoush and S. N. Masoud, Chin. J. Chem., 2011, 29, 1523–1528 CrossRef.
- H. Zamani, Chin. Chem. Lett., 2011, 22, 201–204 CrossRef CAS PubMed.
- Z. M. Wang, D. R. Yuan, Y. S. Yin and G. Su, Opt. Mater., 2007, 29, 663–666 CrossRef CAS PubMed.
- W. Li, Z. W. Liu and S. G. Xiao, Nat. Sci. J. Xiangtan Univ., 2002, 24, 29–32 Search PubMed.
- S. H. Sai, C. M. Nie and X. M. He, J. South China Univ. Technol., Nat. Sci., 2007, 21, 42–45 Search PubMed.
- N. Zhang, W. Z. Shi and S. H. Tang, Henan Science, 2003, 21, 408–410 CrossRef.
- Y. B. Ding, X. Li, T. Li, W. H. Zhu and Y. S. Xie, J. Org. Chem., 2013, 78, 5328–5338 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra15530k |
| This journal is © The Royal Society of Chemistry 2015 |