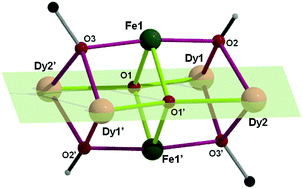
Squashed {Fe2IIIM4III} octahedra (M = Y, Gd, Dy) from the first use of the cyanoacetate ligand in 3d/4f coordination chemistry†
Abstract
The first use of the cyanoacetate ligand in 3d/4f-metal chemistry leads to {Fe2IIIM4III} (M = Dy, Gd, Y) coordination clusters with a squashed octahedral core structure unprecedented in Fe/4f compounds; the DyIII compound exhibits slow relaxation of the magnetisation.

 Please wait while we load your content...
Please wait while we load your content...