Convenient and efficient synthesis of disubstituted piperazine derivatives by catalyst-free, atom-economical and tricomponent domino reactions†
Abstract
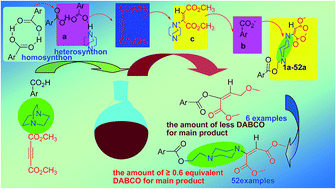
One-pot, atom-economical, catalyst-free and tri-component domino reactions are applied to the diversity-oriented synthesis (DOS) of disubstituted piperazine derivatives under mild conditions with moderate to high yields. This protocol exhibits potential applicability in the synthesis of pharmaceuticals, liquid crystals, complexes, etc. Because of its operational simplicity and convenience, it may be suitable for application in large-scale synthesis.

 Please wait while we load your content...
Please wait while we load your content...