Asymmetric synthesis of amino-benzothiazol derivatives by additions of 2-lithiated benzothiazoles to (S)-N-t-butylsulfinyl-ketimines†
Yanling Daia,
Chen Xiea,
Lingmin Wua,
Haibo Mei*a,
Vadim A. Soloshonokcd,
Jianlin Han*ab and
Yi Panab
aSchool of Chemistry and Chemical Engineering, State Key Laboratory of Coordination Chemistry, Nanjing University, Nanjing, 210093, China. E-mail: meihb@nju.edu.cn; hanjl@nju.edu.cn; Fax: +86-25-83686133; Tel: +86-25-83686133
bInstitute for Chemistry & BioMedical Sciences, Nanjing University, Nanjing, 210093, China
cDepartment of Organic Chemistry I, Faculty of Chemistry, University of the Basque Country UPV/EHU, 20018 San Sebastian, Spain
dIKERBASQUE, Basque Foundation for Science, 48011 Bilbao, Spain
First published on 4th December 2014
Abstract
The reactions between lithium-benzothiazoles and (S)-N-tert-butanesulfinylketimines have been found to be of general synthetic importance for asymmetric preparation of previously unknown type of amino-benzothiazol derivatives of high pharmaceutical potential. In most cases, the reactions proceed with excellent diastereoselectivities and good isolated yields of the target compounds rendering the developed procedure of high synthetic value and immediate practical use.
Introduction
The chemistry and biological study of benzothiazole derivatives is one of the well-developed areas of a most successful interface between organic and medicinal chemistry.1 Number of compounds possessing anti-cancer,2 -microbial,3 -convulsant,4 -diabetic,5 -tubercular,6 -viral,7 -inflammatory,8 -leishmanial9 and -oxidant10 activity, have been identified and introduced to the pharmaceutical market. However, despite quite intensive research activity in this field, one particular class of benzothiazole derivatives of general structure 1 (Scheme 1) remains synthetically unrepresented and biologically unexplored.Compounds of type 1, bearing a biologically important amino group directly attached to the quaternary stereogenic carbon, possess multiple points of functional variation and might possibly be of high pharmaceutical potential. Structurally this type of compounds is rather stereochemically congested and therefore its preparation in enantiomerically pure form would present some synthetic challenge. One can envision that structures of type 1 should be assembled by the corresponding stereo-controlled Mannich addition reactions between properly activated methyne centre in the thiazole ring of benzothiazoles 2 (Scheme 1) and appropriate ketimines. However, this kind of additions is virtually unstudied. Thus, there is only a single literature report on the reactions of lithiated benzothiazoles 2 with racemic N-tert-butanesulfinyl cyclic ketimines 3 resulting in a formation of amino-derivatives 4, used as key intermediates in the development of potent dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes.11 Another relevant example, the additions of benzothiazoles 2 to N-tert-butanesulfinyl aldimines 5, was recently reported by our group.12a In this case, the reactions proceeded with quite unexpected outcome giving rise to the (E)-1,2-bis(2-benzothiazolyl)ethanes 6 instead of the expected Mannich addition products. These very limited and inconclusive literature data clearly indicate that the reactions of benzothiazoles 2 with N-sulfinyl imines deserve a focused, systematic study as a potential approach to a novel type of amino-compounds 1. In particular, such key issues as the stereochemical outcome, generality and practicality of this approach must be answered with a full experimental study. In this work, we described the asymmetric addition reactions between (S)-N-t-butylsulfinyl-ketimines 7 and various substituted benzothiazoles 2. We demonstrate that these reactions take place with excellent stereocontrol and chemical yields rendering this method as highly generalized and synthetically valuable approach for preparation of novel derivatives 1 in enantiomerically pure form.
Results and discussion
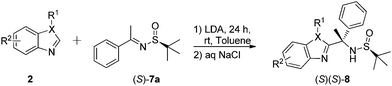
Based on our experience in the Mannich addition reaction of (S)-N-t-butylsulfinyl-ket-13 and -aldimines14–16 we first did the reaction between unsubstituted benzothiazole 2 and imine 7 (Table 1). Application of n-BuLi as a base, to generate the corresponding lithium derivative from 2a, allowed preparation of the target product 8a, albeit in low 30% yield (entry 1). However, the reaction conducted in THF at −78 °C, proceeded with excellent stereocontrol and compound 8a was isolated as a single diastereomer. The use of more sterically demanding LDA, instead of n-BuLi, resulted in preparation of 8a with improved yield and the same excellent diastereoselectivity (entry 2). Further increase in the steric bulk of lithiating reagents, such as LiHMDS (entry 3), NaHMDS (entry 4) or (CH3)3COLi (entry 5) and (CH3)3COK (entry 6), gave rather negative results, rendering LDA as the optimal base to be used in the rest of this study. Further attempts to increase the yield of product 8a, using 1.7 (entry 7) and 2.0 equivalent excess of starting 2a, gave satisfactory result and diastereomerically pure 8a was isolated with up to 71% yield (entry 8). On the other hand, variation of the reaction temperature was less successful (entry 9). Application of various solvents for the reaction of 2a with (S)-7a, allowed us to identify toluene as the optimal reaction medium (entries 10–13). We found, that the reactions initiated at −78 °C and allowed to gradually warm up to ambient temperature provided high chemical yields of target product 8a (entries 11–13).| Entry | Base | 2a (equiv.) | Solvent | Time (h) | T (oC) | Yieldb (%) | Drc |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: ketimine (S)-7a (0.5 mmol), base (1.2 equiv. of 2a) and solvent (5 mL).b Isolated yields.c Determined on crude reaction mixtures by 1H NMR analysis.d No reaction.e Not determined. | |||||||
| 1 | n-BuLi | 1.2 | THF | 9 | −78 | 30 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | LDA | 1.2 | THF | 9 | −78 | 46 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | LiHMDS | 1.2 | THF | 9 | −78 | Trace | NDe |
| 4 | NaHMDS | 1.2 | THF | 9 | −78 | Trace | ND |
| 5 | (CH3)3COLi | 1.2 | THF | 9 | −78 | NRd | — |
| 6 | (CH3)3COK | 1.2 | THF | 9 | −78 | Trace | ND |
| 7 | LDA | 1.7 | THF | 9 | −78 | 70 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | LDA | 2.0 | THF | 9 | −78 | 71 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | LDA | 1.7 | THF | 24 | −78 to r.t. | 61 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | LDA | 2.0 | Toluene | 9 | −40 | 83 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | LDA | 1.7 | Toluene | 12 | −78 to r.t. | 68 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | LDA | 1.7 | Toluene | 18 | −78 to r.t. | 88 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13 | LDA | 1.7 | Toluene | 24 | −78 to r.t. | 96 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Having optimized the reaction conditions, the substrate scope of benzothiazole was studied, and the results were collected in Table 2. First, we tried the reactions between a series of halogen-substituted benzothiazoles 2b–d and ketimine (S)-7a. The steric bulk of the halogen atom seemed not to be of great importance for the stereochemical outcome (entries 2–4), even the presence of fluorine atom (entry 2) had a negative effect on the diastereoselectivity. Similar results were obtained in the reactions of benzothiazoles 2e (entry 5) and 2f (entry 6) bearing ethyl and methoxy groups respectively. On the other hand, the ethoxy-containing benzothiazole 2g (entry 7) gave the target product 8g with a bit lower diastereoselectivity but in good chemical yield. The reaction of benzothiazole 2h (entry 8), bearing bulky t-butyl group, with ketimine (S)-7a proceeded surprisingly well and the corresponding product 8h was isolated in good yield and reasonably good diastereoselectivity, considering the steric bulk of the substituent. Then, one example of 4-substituted benzo[d]thiazole (2i) has been examined as substrate for this reaction, which also proceeded smoothly to give the desired product in 45% yield and excellent dr (entry 9). Finally, the similar heterocycles, such as benzoxazole (2j) and 1-substituted benzimidazole (2k) have been tried as substrates in this reaction. However, almost no corresponding products were obtained (entries 10 and 11).
| Entry | R1 | R2 | X | Product | Yieldb (%) | Drc |
|---|---|---|---|---|---|---|
| a Reaction conditions: 2 (0.85 mmol), ketimine (S)-7a (0.5 mmol), base (1.2 equiv. of 2) and toluene (5 mL).b Isolated yields.c Determined on crude reaction mixtures by 1H NMR analysis.d No detect.e No reaction. | ||||||
| 1 | — | H | S | 8a | 96 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | — | 6-F | S | 8b | 85 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| 3 | — | 6-Cl | S | 8c | 80 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 4 | — | 6-Br | S | 8d | 91 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| 5 | — | 6-Et | S | 8e | 82 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 6 | — | 6-OCH3 | S | 8f | 92 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 7 | — | 6-OEt | S | 8g | 94 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 8 | — | 6-t-Bu | S | 8h | 60 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
| 9 | — | 4-CH3 | S | 8i | 45 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | — | H | O | 8j | Trace | N.d.d |
| 11 | CH3 | H | N | 8k | NRe | — |
Our next objective was to examine the substrate scope of ketimines 7. Varieties of ketimines bearing substituents on the phenyl ring were tried in this system, and the results were shown in Table 3. We began this part of the study with the reactions of a series of halogen-substituted ketimines (S)-7b–d (entries 2–4). Gratifyingly, neither the position nor size of the halogen atom had any effect on the diastereoselectivity (entries 2–4 vs. 1) and the target products 9b–d were isolated as single diastereomers. However, the chemical yields were a bit lower as compared with that of the unsubstituted ketimine (S)-7a reaction (entry 1). Furthermore, the electronic properties of the substituents also had no effect on the perfect stereocontrol in these addition reactions. For example, ketimines (S)-7e–g, bearing methyl (entry 5), trifluoromethyl (entry 6) and methoxy (entry 7) groups, all gave the corresponding products 9e–g with perfect diastereoselectivity (>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Finally, the presence of a heterocyclic moiety in the place of the phenyl was studied using 2-thienyl containing ketimine (S)-7h. The reaction (entry 8) proceeded quite normally, affording the product 9h with excellent stereoselectivity and reasonably good (68%) chemical yield. Thus, quite remarkably, the position as well as the electronic properties of substituents on the starting ketimines (S)-7a–j did not show any effect on the diastereoselectivity of the addition reactions with benzothiazole 2a. Only for the case of 7i, a slight lower diastereoselectivity was observed (96
1). Finally, the presence of a heterocyclic moiety in the place of the phenyl was studied using 2-thienyl containing ketimine (S)-7h. The reaction (entry 8) proceeded quite normally, affording the product 9h with excellent stereoselectivity and reasonably good (68%) chemical yield. Thus, quite remarkably, the position as well as the electronic properties of substituents on the starting ketimines (S)-7a–j did not show any effect on the diastereoselectivity of the addition reactions with benzothiazole 2a. Only for the case of 7i, a slight lower diastereoselectivity was observed (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 dr, entry 9). The noticeable difference in these reactions was the isolated yield of products 9a–j ranging from 58 to 96%.
4 dr, entry 9). The noticeable difference in these reactions was the isolated yield of products 9a–j ranging from 58 to 96%.
| Entry | Ar | Product | Yieldb (%) | Drc |
|---|---|---|---|---|
| a Reaction conditions: benzo[d]thiazole 2a (0.85 mmol), ketimine (S)-7 (0.5 mmol), base (1.2 equiv. of 2a) and toluene (5 mL).b Isolated yields.c Determined on crude reaction mixtures by 1H NMR analysis. | ||||
| 1 | C6H5 | 9a | 96 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | 4-F–C6H4 | 9b | 88 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 3-Cl–C6H4 | 9c | 79 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | 3-Br–C6H4 | 9d | 83 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 4-CH3–C6H4 | 9e | 78 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 3-CF3–C6H4 | 9f | 75 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | 4-MeO–C6H4 | 9g | 88 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | 2-Thienyl | 9h | 68 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | 2-F–C6H4 | 9i | 58 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 10 | 3-CH3–C6H4 | 9j | 64 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
With substrate-generalization part of this study being concluded we proceeded next to the determination of the absolute configuration of products and elucidation of the obtained stereochemical outcome. Taking advantage of high crystallinity of product 9h we conducted its crystallographic analysis, which revealed the corresponding (Ss)(2S) absolute configuration (Fig. 1). The absolute configuration of all other products 8a–i and 9a–j was assigned as (Ss)(2S) based on obvious similarity of their spectral data and chiroptical properties.
Taking into account the (Ss)(2S) preference for absolute configuration of diastereomeric products 8 and 9, we can discuss a plausible mechanistic rationale for the observed stereochemical outcome in the reactions under study. Transition states (TSs) A–C leading to (Ss)(2S) configured products are presented in Fig. 2. Building TSs A–C we considered the fact that ketimines (S)-7 exist and react exclusively in the (E)-geometric configuration.17 To account for the observed reactivity, the effect of the substituents and the absolute configuration of products, we can suggest that transition state TS B as seems to be the most realistic. For instance, in the TS A the phenyl rings of lithium-benzothiazole and ketimine are overlapped rendering this disposition sterically unfavourable. Furthermore, in the case TS A is realized, one should observe some effects of the substituents on both aromatic rings. However, according to the experimental data, only substitution on the benzothiazole ring has noticeable effect on the stereochemical outcome. By contrast, in TSs B and C the aromatic ring of the ketimine has no steric interactions and therefore perfectly agrees with the experimental data showing that substitution pattern on the phenyl (2-thinyl) has no effect on the diastereoselectivity observed. Nevertheless, comparing TSs B and C one might agree that in TS B the lithium-benzothiazole is overlapped only with Me group, while in TS C the steric interactions in question involve the whole N–S–O fragment. Furthermore, in TS B, the ketimine nitrogen and the lithium atoms are in close proximity to each other and could produce the resulting N–Li species with minimum charge separation.18
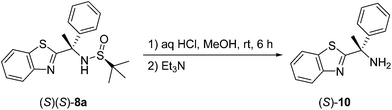
As the final synthetic effort in this study and considering the novelty of products obtained, we decided to provide an example of N-sulfinyl group deprotection and preparation of free amino compounds. As shown in Scheme 2, compound (S)(S)-8a was cleanly deprotected using standard conditions19 to afford the target free amine (S)-10, isolated in good (83%) chemical yield. Also, the enantioselectivity of (S)-10 has been examined and the results obtained confirmed the enantiomeric purity if this compound, indicating that no racemization take place during the deprotection procedure.
Conclusions
In conclusion, the work presented here clearly demonstrates, that the lithium-benzothiazole nucleophiles readily react with (S)-N-tert-butanesulfinylketimines giving rise to the corresponding (S)-1-(benzo[d]thiazol-2-yl)-1-phenylethanamines, the previously unknown type of biologically relevant benzothiazol derivatives. The reactions are shown to be quite general tolerating various substituents on the both reaction partners. In particular, neither the position nor size of a substituent on the aromatic ring of starting ketimines had any effect on the perfect (>99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) diastereoselectivity observed in these reactions. On the other hand, the presence of a substituent on starting benzothiazoles led to some lower stereocontrol ranging from 88
1) diastereoselectivity observed in these reactions. On the other hand, the presence of a substituent on starting benzothiazoles led to some lower stereocontrol ranging from 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 to 98
12 to 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratios of the diastereomeric products. The experimental data reported here clearly suggest that the developed method is of high synthetic value as a general approach for preparation of a variety of novel biologically interesting amino-benzothiazole derivatives.
2 ratios of the diastereomeric products. The experimental data reported here clearly suggest that the developed method is of high synthetic value as a general approach for preparation of a variety of novel biologically interesting amino-benzothiazole derivatives.
Experimental
General information
All imine addition reactions were performed in oven-dried vials under N2 atmosphere. Solvent was dried and distilled prior to use. Benzo[d]thiazoles were synthesized according to literature.20 LDA (2 M in THF) was from Aldrich. These and other chemicals were used as obtained from commercial sources without further purification. Flash chromatography was performed using silica gel 60 (200–300 mesh). Thin layer chromatography was carried out on silica gel 60 F-254 TLC plates of 20 cm × 20 cm. Melting points are uncorrected. Values of optical rotation were measured on a Rudolph Automatic Polarimeter A21101. Infrared spectra were obtained on a Bruker Vector 22 in KBr pellets. 1H, 13C and 19F NMR spectra were recorded on a Bruker AVANCE400M spectrometer. HRMS were conducted on an Agilent 6540Q-TOF LC/MS equipped with an electrospray ionization (ESI) probe operating in positive or negative ion mode.Typical procedure for asymmetric addition of sulfinylimine
Into an oven-dried reaction vial flushed with N2 were taken 2 (0.85 mmol) and anhydrous toluene (3.0 mL). The reaction vial was cooled to −78 °C and LDA (2 M in THF, 0.51 mL) was added dropwise with stirring. After 1 h at −78 °C, sulfinylimine 7 (0.5 mmol) dissolved in anhydrous toluene (2.0 mL) was added dropwise. Stirring was continued for 24 h with the temperature rising to rt. Then the reaction was quenched with saturated NaCl (3.0 mL). The organic layer was taken and the aqueous layer was extracted with EtOAc (2 × 20 mL). The combined organic layers were dried with anhydrous Na2SO4, filtered and the solvent was removed to give the crude product 8 or 9, which was purified by column chromatography (hexane–EtOAc, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
General procedure for deprotection
8a (0.5 mmol) and MeOH (5 mL) were placed in a 25 mL round bottom flask and aq HCl (36%, 1 mL) was added. The reaction was stirred at rt for 6 h, during which the cleavage was monitored by TLC. Volatiles were removed under reduced pressure. The residue was dissolved in CH2Cl2 (10 mL) and Et3N (15 mmol) was added. The mixture was stirred at rt for 1 h, then H2O (10 mL) was added. The organic layer was taken, washed with H2O (2 × 10 mL), dried with anhydrous Na2SO4, filtered and the solvent was removed to give the crude product, which was purified by column chromatography (hexane–EtOAc, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the corresponding deprotection product 10.
1) to afford the corresponding deprotection product 10.
Notes and references
- (a) T. D. Bradshaw and A. D. Westwell, Curr. Med. Chem., 2004, 11, 1009–1023 CrossRef CAS; (b) T. J. Eckroat, A. S. Mayhoub and S. Garneau-Tsodikova, Beilstein J. Org. Chem., 2013, 9, 1012–1044 CrossRef CAS PubMed; (c) S. Y. Ke, Y. H. Wei, Z. W. Yang, K. M. Wang, Y. Liang and L. Q. Shi, Bioorg. Med. Chem. Lett., 2013, 23, 5131–5134 CrossRef CAS PubMed; (d) M. Ban, H. Taguchi, T. Katsushima, M. Takahashi, K. Shinoda, A. Watanabe and T. Tominaga, Bioorg. Med. Chem., 1998, 6, 1069–1076 CrossRef CAS; (e) Y. Liu, Y. Wang, G. Q. Dong, Y. Q. Zhang, S. C. Wu, Z. Y. Miao, J. Z. Yao, W. N. Zhang and C. Q. Sheng, MedChemComm, 2013, 4, 1551–1561 RSC.
- (a) Z. Wang, X. Shi, J. Wang, T. Zhou, Y. Z. Xu, T. T. Huang, Y. F. Li, Y. L. Zhao, L. Yang, S. Y. Yang, L. T. Yu and Y. Q. Wei, Bioorg. Med. Chem. Lett., 2011, 21, 1097–1101 CrossRef CAS PubMed; (b) R. M. Kumbhare, T. Dadmal, U. Kosurkar, V. Sridhar and J. V. Rao, Bioorg. Med. Chem. Lett., 2012, 22, 453–455 CrossRef CAS PubMed; (c) C. G. Mortimer, G. Wells, J. P. Crochard, E. L. Stone, T. D. Bradshaw, M. G. G. Stevens and A. D. Westwell, J. Med. Chem., 2006, 49, 179–185 CrossRef CAS PubMed.
- (a) C. Sheng, J. Zhu, W. Zhang, M. Zhang, H. Ji, Y. Song, H. Xu, J. Yao, Z. Miao, Y. Zhou, J. Zhu and J. Lü, Eur. J. Med. Chem., 2007, 42, 477–486 CrossRef CAS PubMed; (b) B. S. Soni, M. Ranawat, R. Sharma, A. Bhandari and S. Sharma, Eur. J. Med. Chem., 2010, 45, 2938–2942 CrossRef CAS PubMed; (c) P. K. Sahu, P. K. Sahu, S. K. Gupta, D. Thavaselvam and D. D. Agarwal, Eur. J. Med. Chem., 2012, 54, 366–378 CrossRef CAS PubMed.
- (a) P. Jimonet, F. Audiau, M. Barreau, J. C. Blanchard, A. Boireau, Y. Bour, M. A. Coléno, A. Doble, G. Doerflinger, C. Do Huu, M. H. Donat, J. M. Duchesne, P. Ganil, C. Guérémy, E. Honoré, B. Just, R. Kerphirique, S. Gontier, P. Hubert, P. M. Laduron, J. Le Blevec, M. Meunier, J. M. Miquet, C. Nemecek, M. Pasquet, O. Piot, J. Pratt, J. Rataud, M. Reibaud, J. M. Stutzmann and S. Mignani, J. Med. Chem., 1999, 42, 2828–2843 CrossRef CAS PubMed; (b) A. Rana, N. Siddiqui, S. A. Khan, S. E. Haque and M. A. Bhat, Eur. J. Med. Chem., 2008, 43, 1114–1122 CrossRef CAS PubMed; (c) N. Siddiqui, A. Rana, S. A. Khan, S. E. Haque and M. A. Bhat, Bioorg. Med. Chem. Lett., 2007, 17, 4178–4182 CrossRef CAS PubMed; (d) N. D. Amnerkar and K. P. Bhusari, Eur. J. Med. Chem., 2010, 45, 149–159 CrossRef CAS PubMed.
- (a) H. Moreno-Díaz, R. Villalobos-Molina, R. Ortiz-Andrade, D. Díaz-Coutiño, J. L. Medina-Franco, S. P. Webster, M. Binnie, S. Estrada-Soto, M. Ibarra-Barajas, I. León-Rivera and G. Navarrete-Vázquez, Bioorg. Med. Chem. Lett., 2008, 18, 2871–2877 CrossRef PubMed; (b) V. S. Patil, K. P. Nandre, S. Ghosh, V. J. Rao, B. A. Chopade, B. Sridhar, S. V. Bhosale and S. V. Bhosale, Eur. J. Med. Chem., 2013, 59, 304–309 CrossRef CAS PubMed; (c) A. Ammazzalorso, A. Giancristofaro, A. D'Angelo, B. D. Filippis, M. Fantacuzzi, L. Giampietro, C. Maccallini and R. Amoroso, Bioorg. Med. Chem. Lett., 2011, 21, 4869–4872 CrossRef CAS PubMed.
- (a) G. A. Pereira, A. C. Massabni, E. E. Castellano, L. A. S. Costa, C. Q. F. Leite, F. R. Pavan and A. Cuin, Polyhedron, 2012, 38, 291–296 CrossRef CAS PubMed; (b) V. N. Telvekar, V. K. Bairwa, K. Satardekar and A. Bellubi, Bioorg. Med. Chem. Lett., 2012, 22, 649–652 CrossRef CAS PubMed; (c) L. Katz, J. Am. Chem. Soc., 1953, 75, 712–714 CrossRef CAS; (d) Y. Cho, T. R. Ioerger and J. C. Sacchettini, J. Med. Chem., 2008, 51, 5984–5992 CrossRef CAS PubMed.
- S. R. Nagarajan, G. A. De Crescenzo, D. P. Getman, H. F. Lu, J. A Sikorski, J. L. Walker, J. J. McDonald, K. A. Houseman, G. P. Kocan, N. Kishore, P. P. Mehta, C. L. Funkes-Shippy and L. Blystone, Bioorg. Med. Chem., 2003, 11, 4769–4777 CrossRef CAS PubMed.
- (a) S. Shafi, M. M. Alam, N. Mulakayala, C. Mulakayala, G. Vanaja, A. M. Kalle, R. Pallu and M. S. Alam, Eur. J. Med. Chem., 2012, 49, 324–333 CrossRef CAS PubMed; (b) A. Geronikaki, D. Hadjipavlou-Litina and M. Amourgianou, Farmaco, 2003, 58, 489–495 CrossRef CAS.
- F. Delmas, A. Avellaneda, C. D. Giorgio, M. Robin, E. D. Clercq, P. Timon-David and J. P. Galy, Eur. J. Med. Chem., 2004, 39, 685–690 CrossRef CAS PubMed.
- (a) T. Tzanova, M. Gerova, O. Petrov, M. Karaivanova and D. Bagrel, Eur. J. Med. Chem., 2009, 44, 2724–2730 CrossRef CAS PubMed; (b) D. Cressier, C. Prouillac, P. Hernandez, C. Amourette, M. Diserbo, C. Lion and G. Rima, Bioorg. Med. Chem., 2009, 17, 5275–5284 CrossRef CAS PubMed.
- A. Nitta, H. Fujii, S. Sakami, Y. Nishimura, T. Ohyama, M. Satoh, J. Nakaki, S. Satoh, C. Inada, H. Kozono, H. Kumagai, M. Shimamura, T. Fukazawa and H. Kawai, Bioorg. Med. Chem. Lett., 2008, 18, 5435 CrossRef CAS PubMed.
- (a) H. B. Mei, Y. L. Dai, L. M. Wu, V. A. Soloshonok, J. L. Han and Y. Pan, Eur. J. Org. Chem., 2014, 2429–2433 CrossRef CAS . For other examples of the reactions of 5 with lithiated aromatic/heterocyclic compounds affording the expected Mannich products, see: ; (b) H. Mei, C. Xie, L. Wu, V. A. Soloshonok, J. Han and Y. Pan, Org. Biomol. Chem., 2013, 11, 8018–8021 RSC; (c) H. B. Mei, Y. W. Xiong, C. Xie, V. A. Soloshonok, J. L. Han and Y. Pan, Org. Biomol. Chem., 2014, 12, 2108–2113 RSC; (d) C. Xie, H. B. Mei, L. M. Wu, J. L. Han, V. A. Soloshonok and Y. Pan, J. Fluorine Chem., 2014, 165, 67–75 CrossRef CAS PubMed; (e) L. Wu, C. Xie, H. Mei, V. A. Soloshonok, J. Han and Y. Pan, J. Org. Chem., 2014, 79, 7677–7681 CrossRef CAS PubMed; (f) P. Qian, C. Xie, L. M. Wu, H. B. Mei, V. A. Soloshonok, J. L. Han and Y. Pan, Org. Biomol. Chem., 2014, 12, 7909–7913 RSC.
- L. Wu, C. Xie, H. Mei, V. A. Soloshonok, J. L. Han and Y. Pan, Org. Biomol. Chem., 2014, 12, 4620–4627 CAS.
- For the reactions with ketone-derived enolates, see: (a) H. Mei, Y. Xiong, J. Han and Y. Pan, Org. Biomol. Chem., 2011, 9, 1402–1406 RSC; (b) C. Xie, H. B. Mei, L. M. Wu, V. A. Soloshonok, J. L. Han and Y. Pan, RSC Adv., 2014, 4, 4763–4768 RSC.
- For the reactions with ester-derived enolates, see: (a) N. Shibata, T. Nishimine, E. Tokunaga, K. Kawada, T. Kagawa, A. E. Sorochinsky and V. A. Soloshonok, Chem. Commun., 2012, 48, 4124–4126 RSC; (b) M. V. Shevchuk, V. P. Kukhar, G. V. Roeschenthaler, B. S. Bassil, K. Kawada, V. A. Soloshonok and A. E. Sorochinsky, RSC Adv., 2013, 3, 6479–6484 RSC; (c) N. Shibata, T. Nishimine, N. Shibata, E. Tokunaga, K. Kawada, T. Kagawa, J. L. Aceña, A. E. Sorochinsky and V. A. Soloshonok, Org. Biomol. Chem., 2014, 12, 1454–1462 RSC.
- For other types of nucleophiles, see: (a) G. V. Röschenthaler, V. P. Kukhar, I. B. Kulik, M. Y. Belik, A. E. Sorochinsky, E. B. Rusanov and V. A. Soloshonok, Tetrahedron Lett., 2012, 53, 539–542 CrossRef PubMed; (b) K. V. Turcheniuk, K. O. Poliashko, V. P. Kukhar, A. B. Rozhenko, V. A. Soloshonok and A. E. Sorochinsky, Chem. Commun., 2012, 48, 11519–11521 RSC; (c) T. Milcent, J. Hao, K. Kawada, V. A. Soloshonok, S. Ongeri and B. Crousse, Eur. J. Org. Chem., 2014, 3072–3075 CrossRef CAS; (d) C. Xie, L. Wu, H. Mei, V. A. Soloshonok, J. L. Han and Y. Pan, Tetrahedron Lett., 2014, 55, 5908–5910 CrossRef CAS PubMed.
- (a) G. Liu, D. A. Cogan, T. D. Owens, T. P. Tang and J. A. Ellman, J. Org. Chem., 1999, 64, 1278–1284 CrossRef CAS; (b) M. T. Robak, M. A. Herbage and J. A. Ellman, Chem. Rev., 2010, 110, 3600–3740 CrossRef CAS PubMed.
- (a) V. A. Soloshonok, C. Cai and V. J. Hruby, Tetrahedron Lett., 2000, 41, 135–139 CrossRef CAS; (b) V. A. Soloshonok, C. Cai, V. J. Hruby, L. V. Meervelt and T. Yamazaki, J. Org. Chem., 2000, 65, 6688–6696 CrossRef CAS; (c) V. A. Soloshonok, H. Ueki, R. Tiwari, C. Cai and V. J. Hruby, J. Org. Chem., 2004, 69, 4984–4990 CrossRef CAS PubMed.
- (a) C. Xie, H. Mei, L. Wu, V. A. Soloshonok, J. Han and Y. Pan, Eur. J. Org. Chem., 2014, 1445–1451 CrossRef CAS; (b) C. Xie, L. Wu, H. Mei, V. A. Soloshonok, J. Han and Y. Pan, Org. Biomol. Chem., 2014, 12, 7836–7843 RSC.
- (a) R. V. Patel, P. Kumari, D. P. Rajani and K. H. Chikhalia, Med. Chem. Res., 2013, 22, 195–210 CrossRef CAS; (b) C. D. Hou, Y. L. Ren, R. Lang, X. X. Hu, C. G. Xia and F. W. Li, Chem. Commun., 2012, 48, 5181–5183 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, full spectroscopic data for compounds 8–10 and copies of 1H NMR and 13C NMR spectra. CCDC 1024268. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra15405c |
| This journal is © The Royal Society of Chemistry 2015 |