Influence of alkoxy tail length on the phase behaviors of side-chain liquid crystalline polymers without the spacer
Bin Ni,
Junqiu Liao,
Sheng Chen* and
Hai-liang Zhang*
Key Laboratory of Polymeric Materials and Application Technology of Hunan Province, Key Laboratory of Advanced Functional Polymer Materials of Colleges, Universities of Hunan Province, College of Chemistry, Xiangtan University, Xiangtan 411105, Hunan Province, China. E-mail: huaxuechensheng@163.com; zhl1965@xtu.edu.cn
First published on 5th January 2015
Abstract
A series of end-on side-chain liquid crystalline polymers (SCLCPs) based on the biphenyl mesogen which were directly attached to the polymer backbone without the flexible spacer, poly(4,4′-alkoxybiphenylyl methacrylate) (PMBi-m, m = 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 14, 16) were successfully synthesized by free radical polymerization. The chemical structures of the monomers were confirmed by 1H NMR and mass spectrometry. The molecular characterizations of the polymers were performed with 1H NMR, GPC and TGA. The phase behaviors were investigated by a combination of techniques including DSC, POM and 1D/2D WAXD. The experimental results showed that the alkoxy tail played an important role in the phase behaviors of the SCLCPs without the spacer. Firstly, all polymers form the smectic phase. Secondly, the clearing temperatures decrease with a small odd–even effect as the length of the alkyl tail increases and then increase slightly. Lastly, compared with the influence of the alkyl spacer length on liquid crystal properties of end-on SCLCPs with the biphenyl mesogen, the end-on SCLCPs without the spacer and with the different alkyl tail lengths (PMBi-m) exhibit a high glass transition temperature and a stable LC phase.
1. Introduction
Side-chain liquid crystalline polymers (SCLCPs) have been extensively studied not only because of their potential for materials in many fields, such as optical data storage, optic, electro-optic, and nonlinear optic devices, photomechanical applications, etc., but also because they provide a testing challenge to our understanding of the structural factors that promote liquid crystallinity in a polymeric system.1–14It is well known that the properties of SCLCPs depend on the nature of the polymer backbone, the nature of the mesogenic groups and on the length of the flexible spacers.15–17 For the flexible spacer, Ringsdorf and Finkelmann pointed out that the flexible spacer inserted between main chain and mesogenic side groups was indispensable in order for a SCLCP to achieve LC phase since it decoupled the interaction of side chain and polymer backbone, which disrupted the ordered packing of the side mesogens.18,19 This “decoupling concept” can explain why a few end-on SCLCPs without the spacer cannot form the LC phase (see Fig. 1)20–22 and has become a useful guideline for the molecular design of SCLCPs.
However, on the basis of relative literatures, there are many end-on SCLCPs without the spacer to exhibit the LC phase (see Fig. 2).20,23–29 Meantime, as can be seen from Fig. 1 and 2, although some SCLCPs without the spacer cannot show the LC phase, when the alkoxy tails attached to the mesogen, the polymer would present the LC phase, such as, P1 and P17, P2 and P15. Thus, the alkyl tail plays a very important role in the formation of LC phase structure for the SCLCPs without spacer, even identical with the flexible spacer. For example, recently, Chen et al. have report the synthesis and characterization of a new end-on side-chain liquid crystalline polymer (SCLCP), poly[4-(4′-alkoxyphenyloxymethylene)styrene], with the flexible rod-like mesogenic side-chain directly attached to the polymer backbone without flexible spacer (see Fig. 2 P21).27 The results indicated that when the length of alkyl tail (m) was 2, 4, 6, the polymers were amorphous. When m = 8, 12 and 16, the polymers could self-assembly into the smectic A phase. All of these indicated that the alkyl tail thought to be a factor influencing the microstructure, and thus the mesomorphic behavior of end-on SCLCPs without the spacer.
In order to design new materials having targeted properties for applications, we should first develop and understand knowledge-based rules relating polymer structure to LC properties. Although there is much data in the literature describing the LC properties of LC materials, there are few studies in which the length alkyl tail is varied in a systematic fashion for a given backbone and mesogenic group.30,31 In the present work, we intend to describe a detail study of the LC behavior of a series of polymethacrylates containing biphenyl side chains without the spacer (PMBi-m, in which m denotes the number of methylene groups in the flexible tail, m = 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 14, 16, see Scheme 1). Biphenyl has been used in this study because it is the most widely used mesogenic core and has been attached to a wide range of backbone types. Such a study of the phase behavior of the complete homologous series would allow for the rational design of new polymers having tailored phase behavior.
2. Experimental
2.1. Materials
Anhydrous tetrahydrofuran (THF) was distilled from sodium benzophenone ketyl under argon and used immediately. 2,2 Azobisisobutyronitrile (AIBN) was freshly recrystallised from methanol. 4,4′-Diphenol (98%, Alfa Aesar) and bromoalkanes together with other reagents and solvents were used as received without further purification.2.2. Synthesis of Monomers
For convenience, the 4,4′-alkoxybiphenylyl methacrylate monomers were named MBi-m (m = 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 14, 16), and the corresponding polymers were named PMBi-m. The synthetic route of monomers MBi-m is shown in Scheme 1. The experimental details are described as follows:![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH3), 3.9–4.1 (t, 2H, –O–CH2–), 5.5–6.5(2d, 2H, CH2
C–CH3), 3.9–4.1 (t, 2H, –O–CH2–), 5.5–6.5(2d, 2H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–), 6.8–7.8 (m, 8H, Ar-H). Mass Spectrometry (MS) (m/z) [M] calcd for C26H34O3, 394.24.; found, 394.353.
C–), 6.8–7.8 (m, 8H, Ar-H). Mass Spectrometry (MS) (m/z) [M] calcd for C26H34O3, 394.24.; found, 394.353.2.3. Synthesis of Polymers
All polymers was obtained by conventional solution radical polymerization (see Scheme 1), typically carried out as described in the following example.The monomer, AIBN and THF were added into a dried reaction tube containing a magnetic stirrer bar. The molar ratio was set with Nmonomer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NAIBN = 100
NAIBN = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the monomer mass concentration was 25%. After three freeze–pump–thaw cycles, the tube was sealed under vacuum. Polymerization was carried out at 75 °C over 12 h. The sample was purified by similarly re-precipitating three times from THF into methanol.
1, and the monomer mass concentration was 25%. After three freeze–pump–thaw cycles, the tube was sealed under vacuum. Polymerization was carried out at 75 °C over 12 h. The sample was purified by similarly re-precipitating three times from THF into methanol.
2.4. Instruments and measurements
3. Results and discussion
3.1. Synthesis and characterization of monomers and polymers
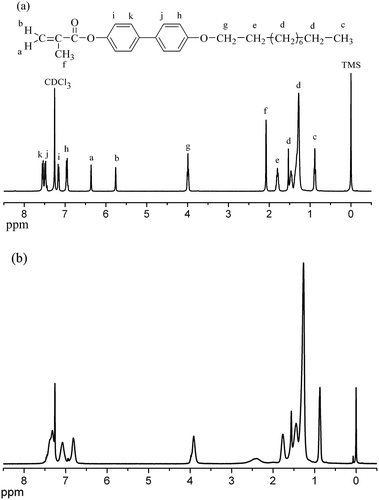
A series of methacrylate biphenyl monomers with the different lengths of alkyl tail (m = 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 14, 16) were synthesized according to the synthetic route illustrated in Scheme 1. All polymers could be easily polymerized by free radical polymerization method. Herein, we use PMBi-10 as an example to elucidate the process. Fig. 3(a) and (b) give the 1H NMR spectra (CDCl3) of the monomer MBi-10 and the polymer PMBi-10, respectively. The MBi-10 showed the characteristic resonances of the vinyl group at 5.71 and 6.42 ppm. In addition, the chemical shifts and peak integrations of all the protons in the monomer are in excellent agreement with its expected structure. Mass spectrometry spectrums further confirmed the molecular structure. After polymerization, these signals disappeared completely. The chemical shifts of PMBi-10 were quite broad and consistent with the expected polymer structure. In order to the influence of Mn on the LC behavior, a series of polymers with the high Mn were synthesized. The Mn of these polymers ranged from 2.6 × 104 to 11.5 × 104 g mol−1, with the polydispersity of 2.0–3.9 were measured by gel permeation chromatography (GPC) (Table 1). Meantime, all polymers showed good thermal stability, that was, the temperatures at 5% weight loss of the samples under nitrogen were about 310 °C measured by TGA at a rate of 20 °C min−1. The molecular characterizations of the polymers are summarized Table 1.| Sample | Mn (×104)a | PDIa | Tgb (°C) | Tic (°C) | T2d (°C) | Tde (N2) |
|---|---|---|---|---|---|---|
| a Relative Mn and PDI were measured by GPC using PS standards.b The glass transition temperatures were measured by DSC at a heating rate of 10 °C min−1 under nitrogen atmosphere during the second heating process.c The transition temperature from liquid crystalline phase to isotropic phase measured by DSC at a heating rate of 10 °C min−1 under nitrogen atmosphere during the second heating process.d The transition temperature from liquid crystalline phase to isotropic phase measured by POM at a heating rate of 10 °C min−1 under nitrogen atmosphere during the second heating process.e The temperatures at 5% weight loss of the samples under nitrogen [Td(N2)] were measured by TGA heating experiments at a rate of 20 °C min−1. | ||||||
| PMBi-1 | 6.5 | 2.1 | — | 295 | 304 | 320 |
| PMBi-2 | 10.3 | 2.1 | — | 277 | 278 | 359 |
| PMBi-3 | 8.5 | 2.4 | — | 288 | 286 | 342 |
| PMBi-4 | 8.2 | 3.6 | — | 275 | 277 | 357 |
| PMBi-5 | 6.2 | 2.0 | — | 266 | 259 | 350 |
| PMBi-6 | 3.4 | 2.5 | 192 | 234 | 240 | 350 |
| PMBi-7 | 10.4 | 2.3 | 202 | 259 | 265 | 325 |
| PMBi-8 | 3.0 | 2.8 | 184 | 221 | 226 | 323 |
| PMBi-9 | 11.5 | 2.4 | 175 | 245 | 250 | 315 |
| PMBi-10 | 2.6 | 3.0 | 144 | 211 | 214 | 342 |
| PMBi-12 | 5.8 | 3.9 | 121 | 217 | 209 | 341 |
| PMBi-14 | 3.8 | 2.3 | 110 | 220 | 219 | 355 |
| PMBi-16 | 6.0 | 3.9 | 96 | 221 | 226 | 362 |
3.2. Phase transitions and phase structures of the polymers
Fig. 4 shows the second heating DSC curves of PMBi-m (m = 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 14, 16) at a rate of 10 °C min−1 under nitrogen atmosphere after eliminating the thermal history. As can be seen from it, a weak second-order transition assigned as a glass transition for PMBi-m (m = 6, 7, 8, 9, 10, 12, 14, 16) and the glass transition temperature (Tg) decreased with the increasing of the alkyl tail length because of the plastification of the alkane (see Fig. 4(b)). In high temperature, all polymers exhibited the board endothermic peak which was marked with the arrows (see Fig. 4(a)). Combined the POM results, these transition peaks corresponded to the interconversion from the LC phase to isotropic phase (the clearing point, Ti) and the results are shown in Table 1. In low temperature, the PMBi-m (m = 12, 14, 16) presented the crystalline melting peaks of the long alkyl tails. In addition, the PMBi-m (m = 1, 2, 3, 4) showed the relatively broad peaks exists left before the clearing point. This phase structure transition process could be detected by POM except for the PMBi-1. For the PMBi-1, the POM and 1D WAXD showed that it only showed the smectic A (SA) phase. Therefore, this relatively broad peak may stand for a possible second order glass transition because this phenomenon can be found in other SCLCPs.32 | ||
| Fig. 4 DSC curves of PMBi-m during the second heating scan at a rate of 10 °C min−1 under nitrogen atmosphere (a) and (b). | ||
POM experiments further study the LC phase structure of the PMBi-m. From the POM experiences, the polymers can be divided into three types. First one is PMBi-1. On cooling from the melt, this polymer showed a smectic schlieren texture (Fig. 5(a)). Continue cooling, no new texture appeared, except that there was a little change of the colour of texture (Fig. 5(b)). The second one is PMBi-m (m = 2, 3, 4). They exhibited two kinds of LC textures and POM results are shown in Fig. 5(c) and (d). After the samples were cooled from the isotropic state to below the isotropic temperature, a smectic schlieren texture was observed, which should correspond to the formation of SA phase at high temperatures (see Fig. 5(c)). Continuing slowly cooling, the new LC texture appeared at 273 °C (see Fig. 5(d)), suggesting the formation of a new LC phase. However, it is hard to estimate the LC phase structure. Second, the PMBi-m (m = 6, 8, 10, 12, 14, 16) only formed the needle-like LC texture below the isotropic temperature and the results were shown in Fig. 5(e)–(h), indicating the formation of SA phase. The POM results were consistent with the DSC results.
1D WAXD analysis can provide useful information concerning molecular arrangement, mode of packing, and type of order in a mesophase of a polymeric LC. The 1D WAXD diffractogram of a powdery sample can be generally divided into the low-angle Bragg reflections at 2θ = 1.5–6° corresponding to the layer spacing of molecular orientational order and the high-angle peaks at 2θ = 20° associated with the two-dimensional liquidlike intermesogenic organization within the layers. To be consistent with the DSC results, the samples were heated to the isotropic temperature, and then slowly cooled to the room temperature. 1D WAXD experiment results showed that the polymers could be divided into four types.
The first one is PMBi-1 and the 1D/2D WAXD were shown in Fig. 6. Fig. 6(a) presents a set of 1D WAXD patterns of PMBi-1 at different temperatures (from 30 °C to 250 °C) and we found that the missed first-order diffraction could be observed upon our 1D WAXD heating experiments. During the heating experiment, only two sharp diffractions at 10.8° and 16.5° are observed and the scattering vectors of the multiple peaks are found to follow a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Moreover, in the whole temperature, 1D WAXD patterns was the same, showing that the phase structure of polymer was not changed. In 2D WAXD results (Fig. 6(b)), we further identified that the first-order diffraction was located on equator (2θ = 5.3°), confirming the LC phase of SA. Therefore, the PMBi-1 exhibited the stable SA phase below the clearing temperature. However, the absence of the first-order diffraction at low temperatures is ascribed to the possible matching of the electron densities between the center portion of the side-chain sublayer and the main-chain sublayer of the SA structure.33
3. Moreover, in the whole temperature, 1D WAXD patterns was the same, showing that the phase structure of polymer was not changed. In 2D WAXD results (Fig. 6(b)), we further identified that the first-order diffraction was located on equator (2θ = 5.3°), confirming the LC phase of SA. Therefore, the PMBi-1 exhibited the stable SA phase below the clearing temperature. However, the absence of the first-order diffraction at low temperatures is ascribed to the possible matching of the electron densities between the center portion of the side-chain sublayer and the main-chain sublayer of the SA structure.33
 | ||
| Fig. 6 1D WAXD patterns of the PMBi-1 during the heating process of the as-cast film (a) and 2D WAXD patterns of the PMBi-1 at the fiber pattern at the room temperature (b). | ||
The second one is PMBi-2, PMBi-3 and PMBi-4. Fig. 7(a) describes the structurally sensitive 1D WAXD patterns of the PMBi-2 from 50 °C to 280 °C. As can be seen from it, in the low 2θ region, four obvious diffraction peaks are observed, with a ratio of scattering vector q (q = 4π![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/λ, with λ the X-ray wavelength and 2θ the scattering angle) of 1
θ/λ, with λ the X-ray wavelength and 2θ the scattering angle) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7
1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.9
2.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.4. Interesting, the peak at 5.33° is not as broad as those at 3.22°, 9.34°, and 14.17°, meantime, the q-ratio of these four peaks is not equal to 1
4.4. Interesting, the peak at 5.33° is not as broad as those at 3.22°, 9.34°, and 14.17°, meantime, the q-ratio of these four peaks is not equal to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2:3
2:3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, indicating that the polymers exhibit two phase structures. To elucidate the packing of mesogens in the mesophase, the experimental interlayer distance was compared to the lamellar spacing L calculated using molecular modeling software from Material Studios 3.0. (L is the distance between a C-atom of the end-methyl group of the mesogen (full extended) and the carbon atom of the polymer chain.) The layer spacing derived from the Bragg reflection at 2θ = 3.22° (d1 = 2.74 nm) is longer than L (1.65 nm) of one monomer repeat unit of MBi-2, but smaller than 2L, suggesting a tilted bilayer lamellar structure (SmC). While the second peak at 2θ = 5.53° (d2 = 1.61 nm) corresponds well to one monomer repeat unit length (1.65 nm). Therefore, the LC phase structure of PMBi-2 maybe involves both mono- and bilayer packing arrangements, leading to partially responsible for two adjacent transition peaks for the DSC (see Fig. 4) and for the difficulty in growing perfect LC textures (see Fig. 7(c)). This phenomenon has been reported by Tang B. Z.34 2D WAXD further identify the phase structures. The 2D WAXD pattern of PMBi-2 recorded at 25 °C with the X-ray incident beam perpendicular to the fiber direction is shown in Fig. 7(b). In the high-angle region at 21.0°, a pair of strong scattering halos is observed to be concentrated on equator, which is mainly resulted from the interference of side-chains which pack parallel to each other, indicating that the sample is well oriented. On the other hand, in the low-angle region, three low-angle spots, which should be correlated to the smectic layer diffraction, appears on meridian. As the chain backbones are aligned along equator, this diffraction pattern indicates that the mesogenic groups on side chains are parallel to the smectic layer normal. Surprised, in the low-angle region, four rather diffuse spots with 2θ of 3.2° are located in quadrant, which imply some characteristics of an SmC structure.5,33 On the other hand, a pair of streaks can be observed at 9.4°, which can be assigned as the third order diffraction for SmC phase.
4, indicating that the polymers exhibit two phase structures. To elucidate the packing of mesogens in the mesophase, the experimental interlayer distance was compared to the lamellar spacing L calculated using molecular modeling software from Material Studios 3.0. (L is the distance between a C-atom of the end-methyl group of the mesogen (full extended) and the carbon atom of the polymer chain.) The layer spacing derived from the Bragg reflection at 2θ = 3.22° (d1 = 2.74 nm) is longer than L (1.65 nm) of one monomer repeat unit of MBi-2, but smaller than 2L, suggesting a tilted bilayer lamellar structure (SmC). While the second peak at 2θ = 5.53° (d2 = 1.61 nm) corresponds well to one monomer repeat unit length (1.65 nm). Therefore, the LC phase structure of PMBi-2 maybe involves both mono- and bilayer packing arrangements, leading to partially responsible for two adjacent transition peaks for the DSC (see Fig. 4) and for the difficulty in growing perfect LC textures (see Fig. 7(c)). This phenomenon has been reported by Tang B. Z.34 2D WAXD further identify the phase structures. The 2D WAXD pattern of PMBi-2 recorded at 25 °C with the X-ray incident beam perpendicular to the fiber direction is shown in Fig. 7(b). In the high-angle region at 21.0°, a pair of strong scattering halos is observed to be concentrated on equator, which is mainly resulted from the interference of side-chains which pack parallel to each other, indicating that the sample is well oriented. On the other hand, in the low-angle region, three low-angle spots, which should be correlated to the smectic layer diffraction, appears on meridian. As the chain backbones are aligned along equator, this diffraction pattern indicates that the mesogenic groups on side chains are parallel to the smectic layer normal. Surprised, in the low-angle region, four rather diffuse spots with 2θ of 3.2° are located in quadrant, which imply some characteristics of an SmC structure.5,33 On the other hand, a pair of streaks can be observed at 9.4°, which can be assigned as the third order diffraction for SmC phase.
 | ||
| Fig. 7 1D WAXD patterns of the PMBi-2 during the heating process of the as-cast film (a) and 2D WAXD patterns of the PMBi-2 at the fiber pattern at the room temperature (b). | ||
The third one is PMBi-m (m = 5, 6, 7, 8, 9, 10). Fig. 8(a) and (b) illustrate the temperature-variable 1D WAXD patterns of PMBi-6 from 30 to 200 °C and from 200 to 30 °C in the 2θ region from 2.5° to 30°. At low angle, three narrow reflection peaks were observed. The scattering vectors of these peaks are found to follow a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, indicating a smectic structure with a periodicity of 3.22 nm. Compared with the L of MBi-6 (2.15 nm), the polymer formed bilayer smectic A phase, in which the alkyl tails are interdigitated in an antiparallel fashion.
3, indicating a smectic structure with a periodicity of 3.22 nm. Compared with the L of MBi-6 (2.15 nm), the polymer formed bilayer smectic A phase, in which the alkyl tails are interdigitated in an antiparallel fashion.
 | ||
| Fig. 8 1D WAXD patterns of the PMBi-6 during the heating process (a) and the first cooling process (b) of the as-cast film. | ||
The last one is PMBi-12, PMBi-14 and PMBi-16. With increasing the length of alkyl terminals, the crystallization phenomenon of longer alkyl tails could be detected by the 1D WAXD, so 1D WAXD experiments for PMBi-m (m = 12, 14 and 16) provided the different information about the changes of structural relative to the PMBi-m (m = 6, 8 and 10). Herein, we use the PMBi-16 as an example (see Fig. 9(a) and (b)) to elucidate the structural evolution.
At lower temperature range the WAXD patterns only rendered several peaks (as shown in Fig. 9(b)) in the high 2θ region of 17–30°, indicating the typical crystal formed by the long alkoxy tails. In the low 2θ region of 1.5–15°, seven obvious diffraction peaks were observed. The ratio of scattering vectors of these peaks was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2:3
2:3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4:6
4:6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, indicating the polymer formed bilayer smectic A phase. When the sample was heated to 70 °C, several peaks in Fig. 9(b) gradually disappeared, showing the alkyl tail entry into the isotropic phase. Thus, the enantiotropic phase transition sequence of PMBi-16 follows: the bilayer smectic A phase with the crystal of alkyl tail ↔ the bilayer smectic A phase ↔ isotropic phase. Based on the 1D WAXD, the d-spacing values of PMBi-m (m = 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 14, 16) are shown in Table 2. As can be seen from it, the spacing of layers of the polymers increased with the increase of alkyl tail.
8, indicating the polymer formed bilayer smectic A phase. When the sample was heated to 70 °C, several peaks in Fig. 9(b) gradually disappeared, showing the alkyl tail entry into the isotropic phase. Thus, the enantiotropic phase transition sequence of PMBi-16 follows: the bilayer smectic A phase with the crystal of alkyl tail ↔ the bilayer smectic A phase ↔ isotropic phase. Based on the 1D WAXD, the d-spacing values of PMBi-m (m = 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 14, 16) are shown in Table 2. As can be seen from it, the spacing of layers of the polymers increased with the increase of alkyl tail.
| Sample | 2θ (°)a | d-Spacinga (nm) | Calculated length of the mesogenic unitsb (nm) |
|---|---|---|---|
| a Obtained from the 1D WAXD experiments.b Assuming the n-alkoxy tails in the side chains have an all-trans conformation. | |||
| PMBi-1 | 5.30 | 1.67 | 1.51 |
| PMBi-2 | 3.22 | 2.74 | 1.65 |
| PMBi-3 | 3.24 | 2.72 | 1.76 |
| PMBi-4 | 3.20 | 2.78 | 1.91 |
| PMBi-5 | 2.88 | 3.07 | 2.01 |
| PMBi-6 | 2.74 | 3.22 | 2.15 |
| PMBi-7 | 2.51 | 3.52 | 2.26 |
| PMBi-8 | 2.41 | 3.66 | 2.40 |
| PMBi-9 | 2.30 | 3.84 | 2.51 |
| PMBi-10 | 2.25 | 3.92 | 2.66 |
| PMBi-12 | 2.15 | 4.11 | 2.91 |
| PMBi-14 | 2.04 | 4.33 | 3.16 |
| PMBi-16 | 1.93 | 4.58 | 3.41 |
3.3. Phase transitions and phase structures of the polymers
Based on DSC, POM and 1D/2D WAXD experiments results, all polymers formed the smectic phase and the schematic drawing of phase structure of polymers is shown in Fig. 10. For the PMBi-1, the Duran and Gramain et al. have found that it presented the SOB → SA → N → I with the increase of temperature.35,36 However, our polymer only showed the smectic A phase. As for this, it may be caused by the high Mn and the high viscosity of our polymers. Fig. 11 showed the dependence of the transition temperatures (Tg and Ti) on the length of alkyl tail, m, for the PMBi-m series (a) and on the length of spacer, m, for the poly[ω-(4′-methoxybiphenyl-4-yloxy)alkyl methacrylate]s (PMBiA-m) (b).16,37,38 Compared with the phase behavior of PMBiA-m, the PMBi-m presented the high Tg and Ti. As for this, it because that the formation of smetic phase structure was developed by the mesogens and the main chain for the SCLCPs without spacer. For later, the polymers exhibited the smetic phase owing to the decoupling and self-organization of the mesogen moieties. On the other hand, the clearing temperatures decrease with a small odd–even effect as the length of the alkyl tail increases and then increase slightly. When the alkoxy tails were added to the mesogen, the interaction of mesogen can increase due to the dipole–dipole interaction, leading to the formation of LC phase. Meantime, the long flexible alkyl side chains were beneficial to the layered structures due to the unfavorable interaction between mesogens and the apolar alkyl side chains, leading to the stable of LC phase.39,40 | ||
| Fig. 11 Dependence of the glass transition temperatures (Tg) and clearing temperatures (Ti) on the number m of methylene groups in the tail for PMBi-m (a) and in the spacer for PMBiA-m. | ||
4. Conclusions
In summary, we have investigated the phase structures and transitions of a series of SCLCPs (PMBi-m, m = 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 14, 16) with the different alkyl tail lengths via DSC, POM and 1D/2D WAXD. The results showed that the PMBi-m exhibited the stable smectic phase. Through studying, the alkyl tail plays an important role in the formation of LC phase structure for the SCLCPs without the spacer. Meantime, it is easier to synthesize the SCLCPs when the mesogens are introduced into the polymer backbone in comparison with the synthesis of SCLCPs with the spacer, which is of great importance for real applications. At present, our group is having exploited its real applications, such as solid-solid phase transition polymer materials. And this work will be presented in the near future.Acknowledgements
This research was financially supported by the National Nature Science Foundation of China (51373148).References
- N. Gimeno, I. Pintre, M. Martínez-Abadía, J. L. Serrano and M. B. Ros, RSC Adv., 2014, 4, 19694 RSC.
- P. Kohn, L. Ghazaryan, G. Gupta, M. Sommer, A. Wicklein, M. Thelakkat and T. Thurn-Albrecht, Macromolecules, 2012, 45, 5676 CrossRef CAS.
- P. Deshmukh, M. Gopinadhan, Y. Choo, S. Ahn, P. W. Majewski, S. Y. Yoon, O. Bakajin, M. Elimelech, C. O. Osuji and R. M. Kasi, ACS Macro Lett., 2014, 3, 462 CrossRef CAS.
- X. Q. Liu, J. Wang, S. Yang and E. Q. Chen, ACS Macro Lett., 2014, 3, 834 CrossRef CAS.
- Z. Q. Yu, J. W. Y. Lam, C. Z. Zhu, E. Q. Chen and B. Z. Tang, Macromolecules, 2013, 46, 588 CrossRef CAS.
- J. G. Rudick and V. Percec, Acc. Chem. Res., 2008, 41, 1641 CrossRef CAS PubMed.
- A. G. Cook, R. T. Inkster, A. Martinez-Felipe, A. Ribes-Greus, I. W. Hamley and C. T. Imrie, Eur. Polym. J., 2012, 48, 821 CrossRef CAS PubMed.
- A. Martinez-Felipe, Z. Lu, P. A. Henderson, S. J. Picken, B. Norder, C. T. Imrie and A. Ribes-Greus, Polymer, 2012, 53, 2604 CrossRef CAS PubMed.
- B. M. Rosen, C. J. Wilson, D. A. Wilson, M. Peterca, M. R. Imam and V. Percec, Chem. Rev., 2009, 109, 6275 CrossRef CAS PubMed.
- K. G. Gutiérrez-Cuevas, L. Larios-López, R. J. Rodríguez-González, B. Donnio and D. Navarro-Rodríguez, Liq. Cryst., 2013, 40, 534 CrossRef.
- W. Wei, Y. Liu and H. Xiong, Polymer, 2013, 54, 6793 CrossRef CAS PubMed.
- X. F. Chen, Z. H. Shen, X. H. Wan, X. H. Fan, E. Q. Chen, Y. G. Ma and Q. F. Zhou, Chem. Soc. Rev., 2010, 39, 3072 RSC.
- J. F. Ban, S. Chen and H. L. Zhang, RSC Adv., 2014, 4, 54158 RSC.
- J. F. Ban, S. Chen and H. L. Zhang, Polym. Chem., 2014, 5, 6558 RSC.
- T. Ganicz and W. Stańczyk, Materials, 2009, 2, 95 CrossRef CAS PubMed.
- A. A. Craig and C. T. Imrie, Macromolecules, 1995, 28, 3617 CrossRef CAS.
- X. Li, L. Fang, L. Hou, L. Zhu, Y. Zhang, B. Zhang and H. Zhang, Soft Matter, 2012, 8, 5532 RSC.
- H. Finkelmann, H. Ringsdorf and J. H. Wendorff, Makromol. Chem., 1978, 179, 273 CrossRef CAS.
- H. Finkelmann and G. Rehage, Adv. Polym. Sci., 1984, 60, 97 Search PubMed.
- V. Frosini, G. Levita, D. Lupinacci and P. L. Magagnini, Mol. Cryst. Liq. Cryst., 1981, 66, 21 CrossRef.
- E. C. Hsu, S. B. Clough and A. Blumstein, J. Polym. Sci., Polym. Lett. Ed., 1977, 15, 545 CrossRef CAS.
- B. Wang, H. Du and J. Zhang, Steroids, 2011, 76, 204 CrossRef CAS PubMed.
- V. Frosini and P. L. Magagnini, J. Polym. Sci., Polym. Lett. Ed., 1974, 12, 23 CrossRef CAS.
- P. L. Magagnini, A. Marchetiti, F. Matera, G. Pizzirani and G. Turchi, Eur. Polym. J., 1974, 10, 585 CrossRef CAS.
- A. K. Alimoglu and A. Ledwith, Polymer, 1984, 25, 1342 CrossRef CAS.
- Y. S. Lipatov, V. V. Tsukruk and V. V. Shilov, J. Macromol. Sci., Polym. Rev., 1984, 24, 173 CrossRef.
- J. F. Zheng, Z. Q. Yu, X. Liu, X. F. Chen, S. Yang and E. Q. Chen, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 5023 CrossRef CAS.
- R. J. Rodríguez-González, L. Larios-López, D. Navarro-Rodríguez, C. Solano and G. Martínez-Ponce, Mol. Cryst. Liq. Cryst., 2009, 511, 1753 Search PubMed.
- V. P. Shibaev and N. Plat, Adv. Polym. Sci., 1984, 60, 173 CrossRef.
- J. F. Zheng, X. Liu, X. F. Chen, X. K. Ren, S. Yang and E. Q. Chen, ACS Macro Lett., 2012, 1, 641 CrossRef CAS.
- S. Chen, A. Ling and H. L. Zhang, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 2759 CrossRef CAS.
- M. Xie and C. Zhang, Polym. Prepr., 2007, 48, 518 CAS.
- X. Q. Zhu, J. H. Liu, Y. X. Liu and E. Q. Chen, Polymer, 2008, 49, 3103 CrossRef CAS PubMed.
- W. Y. Jacky, L. X. Kong, Y. Dong, K. L. Kevin, C. Xu and B. Z. Tang, Macromolecules, 2000, 33, 5027 CrossRef.
- C. Chovino, D. Guillon and P. Gramain, Polymer, 1998, 39, 6385–6390 CrossRef CAS.
- R. Duran, D. Guillon, P. Gramain and A. Skoulios, J. Phys., 1988, 49, 1455 CAS.
- S. Koltzenburg, D. Wolff, J. Springer and O. Nuyken, J. Polym. Sci., Part A: Polym. Chem., 1998, 36, 2669 CrossRef CAS.
- A. A. Craig and C. T. Imrie, J. Mater. Chem., 1994, 4, 1705 RSC.
- V. Percec and C. Pugh, in Side Chain Liquid Crystal Polymers, ed. C. B. McArdle, Chapman and Hall, New York, 1989, p. 30 Search PubMed.
- C. Pugh and A. L. Kiste, Prog. Polym. Sci., 1997, 22, 601 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |